Electrochemical method for removing nitrate from drinking water source
An electrochemical and nitrate technology, applied in chemical instruments and methods, water/sewage treatment, water/sludge/sewage treatment, etc., can solve the problems of no solution, limit the practical application of electrochemical methods, pollution, etc., and achieve Easy to operate, save secondary treatment costs, and reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
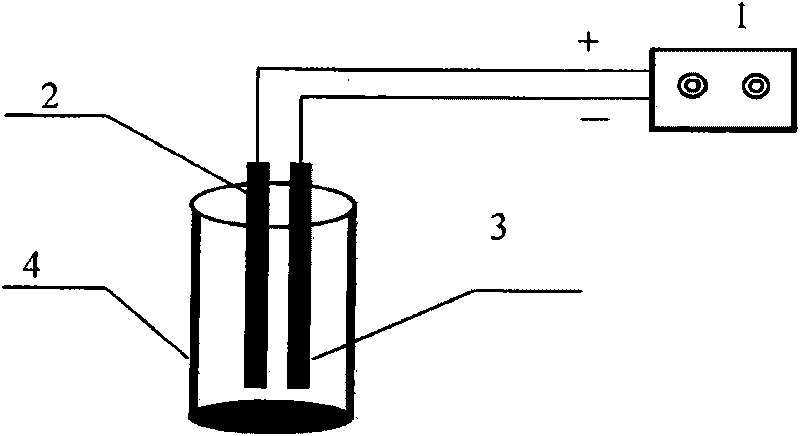
[0045] Such as figure 1 In the device shown, the electrolytic cell is a cylindrical water tank with a volume of 500mL, made of polyethylene, the cathode is Cu / Zn (Cu: 62.2wt%; Zn: 37.8wt%) linear electrode, and the anode is Ti / Zn. Pt-IrO 2 For the wire electrode, a DC voltage stabilizer is used as the power supply, the effective voltage is 0-50V, and the effective current is 0-5A. The volume of nitrate solution for each electrolysis is 400mL, and the effective use area of the electrode is 50cm 2 .
[0046] In the experiment, artificially synthesized nitrate polluted water (NO 3 - -N, 50mg / L; Na 2 SO 4 ; 0.5g / L, NaCl0.5g / L) 400mL is put into the electrolyzer, turn on the power, adjust the electric current, make the electric current density be 40mA / cm 2 . After 5 hours of reaction, the concentration of nitrate nitrogen in the effluent was 6.5mg / L; neither nitrite nor ammonia nitrogen was detected, which met the national drinking water standard.
Embodiment 2
[0048] The method for removing nitrate in water is as in Example 1, but the concentration of nitrate nitrogen in the groundwater increases to 100mg / L. After 5 hours of reaction, the concentration of nitrate nitrogen in the effluent is 9.7mg / L; neither nitrite nor ammonia nitrogen is detected, which meets the requirements of the national standard. drinking water standards.
Embodiment 3
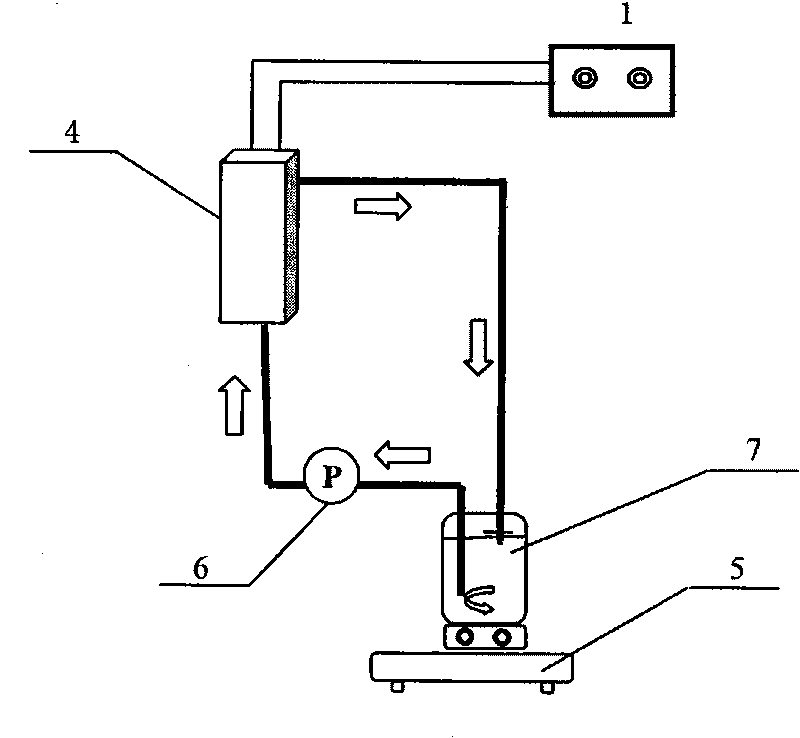
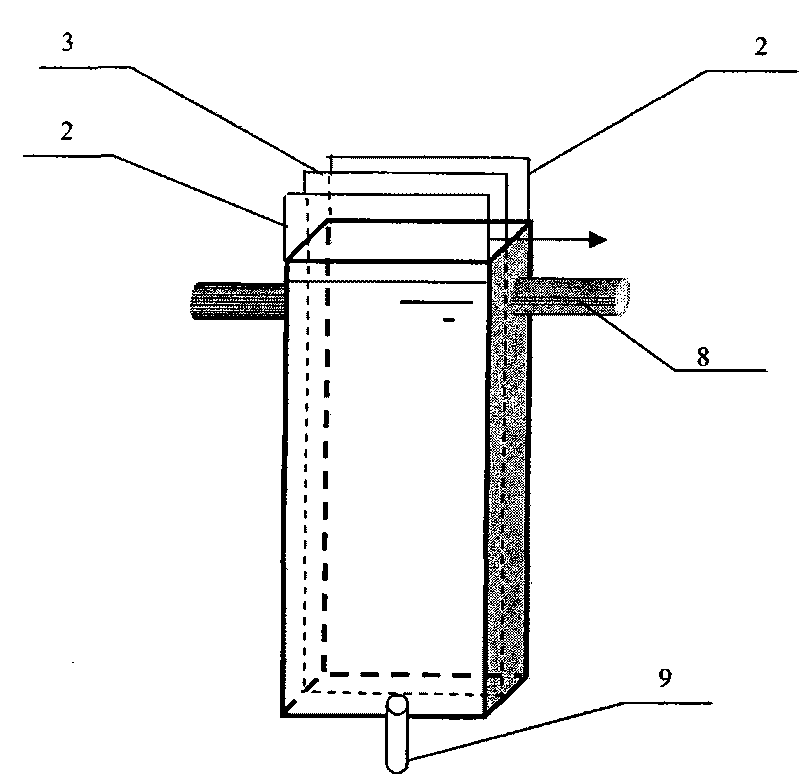
[0050] Such as figure 2 and image 3 As shown, the electrolytic cell (reactor) is a 2cm x 13cm x 5.2cm=135.2cm 3 The cuboid water tank is made of polyethylene, the cathode is Cu / Zn (Cu: 62.2wt%; Zn: 37.8wt%) plate electrode, and the anode is Ti / Pt-IrO 2 Plate electrode, the cathode is placed in the center of the electrolytic cell, and the anode is placed on both sides of the cathode. A DC voltage regulator is used as the power supply with an effective voltage of 0-50V and an effective current of 0-5A. The volume of nitrate solution for each electrolysis is 1L, and the effective use area of the electrode is 10cm x 13cm=130cm 2 . During the electrolysis process, the liquid is circulated between the circulation tank and the reactor through the circulation pump.
[0051] In the experiment, artificially synthesized nitrate polluted water (NO 3 - -N, 25mg / L; Na 2 SO 4 ; 0.5g / L, NaCl0.5g / L) 1L is put into the circulation tank, turn on the power supply, adjust the current ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com