Monocyclic benzoxazine intermediate with low viscosity and a plurality of functional groups and synthesis method thereof
A benzoxazine and multi-functional technology, which is applied in the field of ring-opening polymerization and its polymer preparation, can solve the problems of monocyclic benzoxazine intermediates that cannot be cross-linked and cured, and have low mechanical and thermal properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1, 3-allyl-6-cyano-3,4-dihydro-2H-1, the synthesis of 3-benzoxazine (NB-ala):
[0076] Add 10.0g of p-cyanophenol, 6.5mL of allylamine, 1ml of triethylamine, and 40mL of anhydrous dioxane to a 100mL three-neck flask in sequence, stir to mix, and cool down with an ice-water bath. Slowly add 5g of paraformaldehyde, gradually raise the temperature to 90°C, and react at this temperature for 4h to obtain a yellow benzoxazine intermediate solution. The reaction mixture was rotary evaporated, and the obtained crude product was recrystallized with anhydrous ether to obtain pale yellow crystals with a yield of 67%.
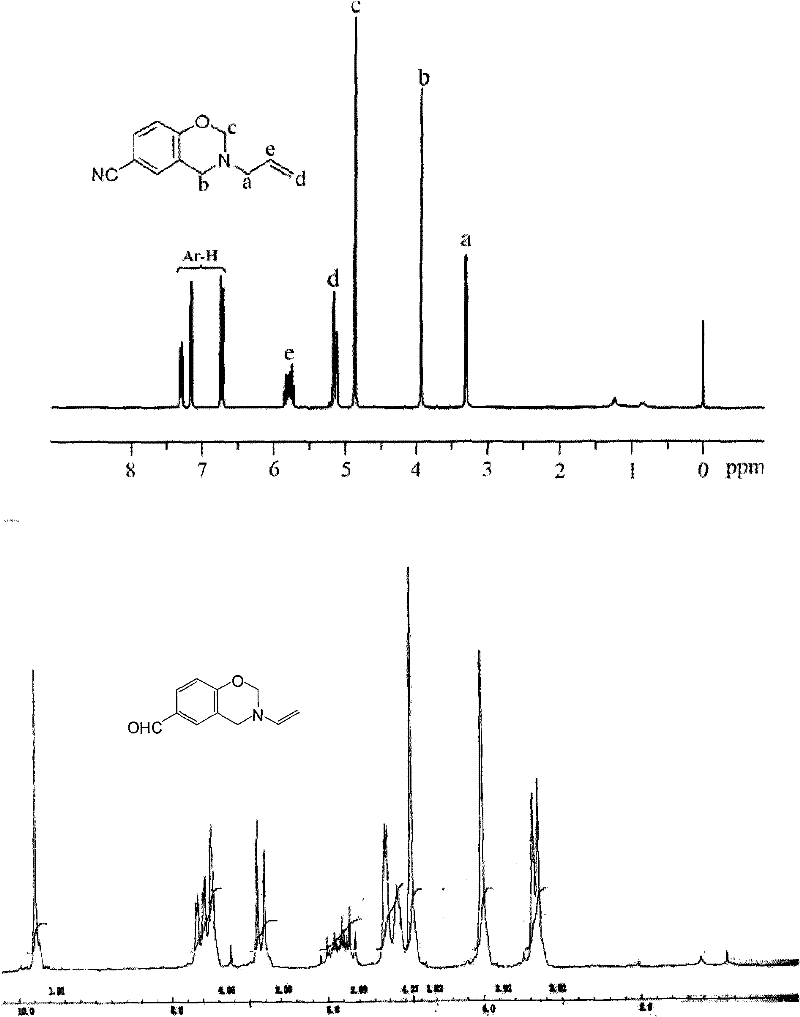
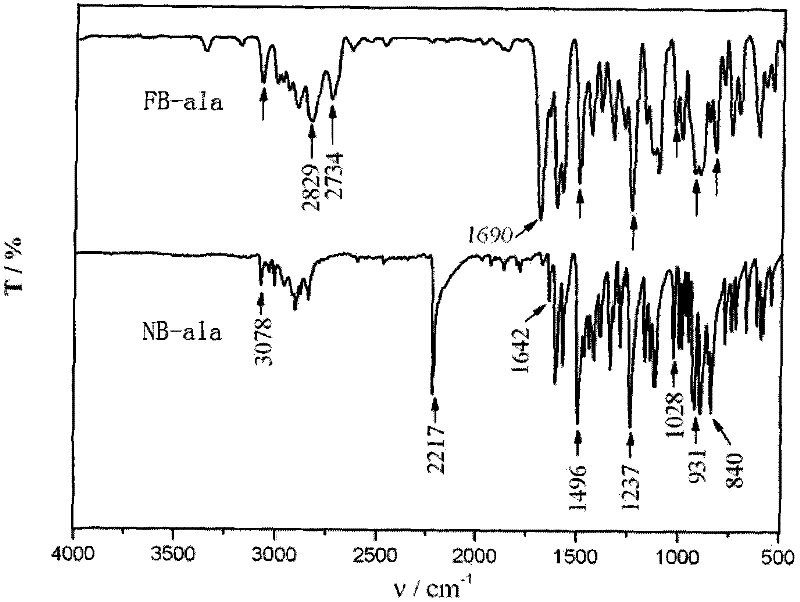
[0077] Elemental Analysis: C 12 h 12 N 2 O, theoretical value %: C, 72.00%; H, 6.00%; N, 14.00%. Analytical value: 0, 71.43%; H, 5.92%; N, 13.77%. 1 H NMR (CDCl 3 , 300MHz, δ): 3.34ppm (d, N-CH 2 -C), 3.99ppm(s, Ar-CH 2 -N), 4.94ppm (s, O-CH 2 -N), 5.19ppm (m, =CH 2 ), 5.86ppm (m, -CH=), 6.81-7.42ppm (Ar-H). FTIR (KBr) v: 1028cm -1 (C-O-C symmet...
Embodiment 2
[0078] Embodiment 2,3-allyl-6-formyl-3,4-dihydro-2H-1, the synthesis of 3-benzoxazine (FB-ala)
[0079] Add 10.2g of p-hydroxybenzaldehyde, 6.5mL of allylamine, 1ml of triethylamine, and 40mL of anhydrous dioxane into a 100mL three-necked flask in sequence, stir to mix and dissolve, and cool down with an ice-water bath. Slowly add 50 g of paraformaldehyde, gradually raise the temperature to 90° C., and react at this temperature for 4 hours to obtain a yellow benzoxazine intermediate solution. The reaction mixture was rotary evaporated, and the obtained crude product was dissolved in 30mL of dichloromethane, washed several times with 1N NaOH aqueous solution and deionized water, and left to separate layers. After the dichloromethane phase was concentrated, column chromatography gave a light yellow transparent Liquid, 72% yield.
[0080] Elemental Analysis: C 12 h 13 NO 2 , Theoretical %: C, 70.94%; H, 6.40%; N, 6.90%. Analytical values: C, 70.31%; H, 6.92%; N, 6.73%. 1 H N...
Embodiment 3
[0081] Example 3, Synthesis of 3-allyl-6-cyano-3,4-dihydro-2H-1,3-benzoxazine (NB-ala): Add 59.5g of para Cyanophenol, 37.5mL allylamine, 6ml triethylamine, 200mL absolute ethanol, stirred and mixed, and cooled with an ice-water bath. Slowly add 30 g of paraformaldehyde, gradually raise the temperature to reflux, and react at reflux temperature for 4 hours to obtain a yellow benzoxazine intermediate solution. The reaction mixture was rotatively evaporated, and the obtained crude product was recrystallized with anhydrous ether to obtain pale yellow crystals with a yield of 72%.
[0082] Elemental Analysis: C 12 h 12 N 2 O, theoretical value %: C, 72.00%; H, 6.00%; N, 14.00%. Analytical value: C, 71.43%; H, 5.92%; N, 13.77%. 1 H NMR (CDCl 3 , 300MHz, δ): 3.34ppm (d, N-CH 2 -C), 3.99ppm(s, Ar-CH 2 -N), 4.94ppm (s, O-CH2-N), 5.19ppm (m, =CH 2 ), 5.86ppm (m, -CH=), 6.81-7.42ppm (Ar-H). FTIR (KBr) v: 1028cm -1 (C-O-C symmetrical telescopic), 1237cm -1 (C-O-C asymmetric tel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com