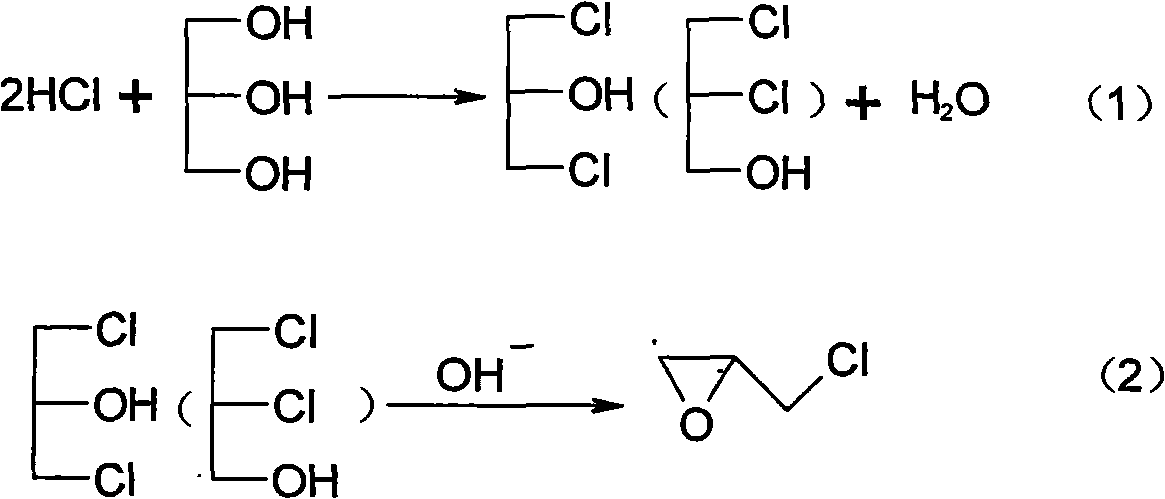
Method for preparing dichlorohydrin and reaction device
A technology of dichloropropanol and a reaction device is applied in the field of a method for preparing dichloropropanol and a reaction device, and can solve the problems of serious environmental pollution, increased reaction process, difficult waste water treatment, etc., and achieves high reaction efficiency, improved reaction efficiency, The effect of improving industrial production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
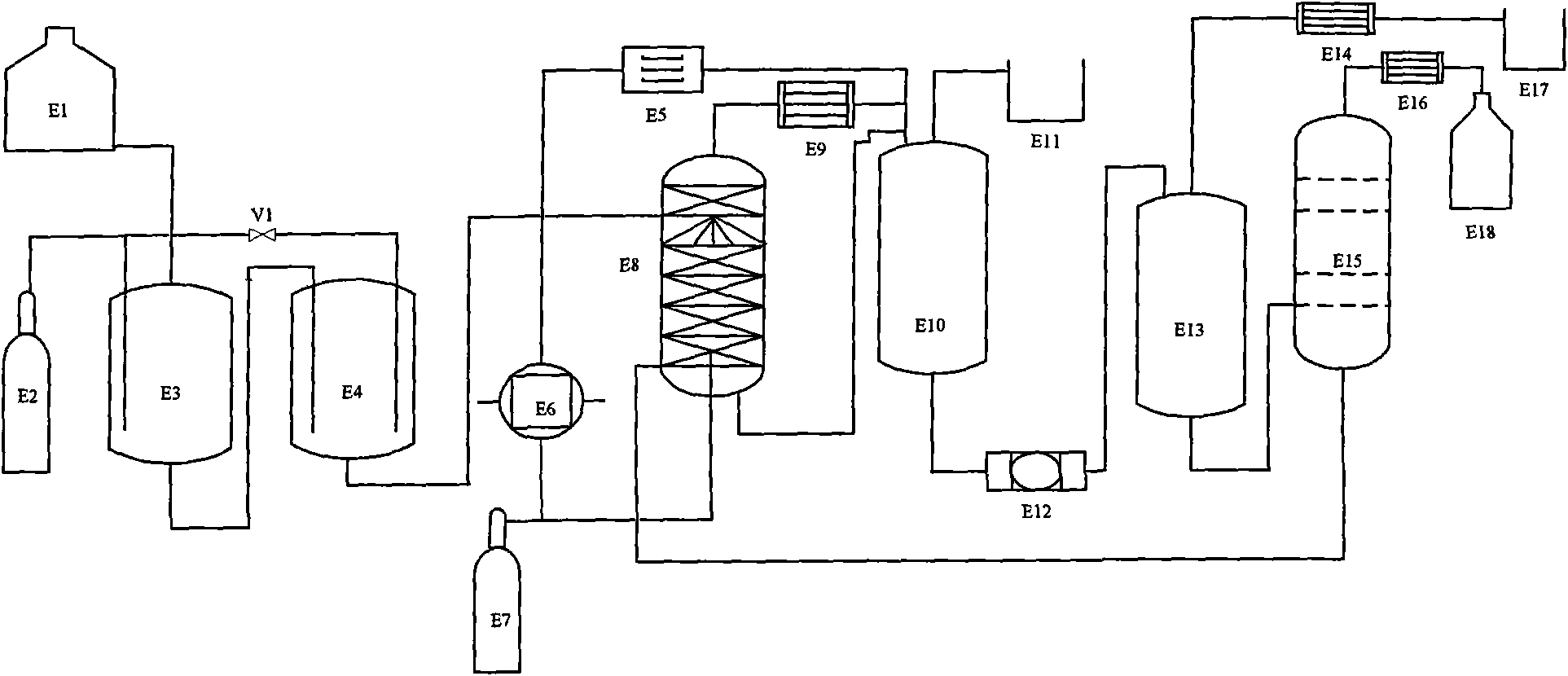
[0028] Embodiment 1: see figure 1 , 2 , feed 93% glycerin in the head tank E1 into the reaction kettle E3, and at the same time feed the acetic acid catalyst accounting for 5% of the glycerin substance, heat to 65°C, and continuously feed HCl gas from the steel cylinder E2 to E3, and the speed of HCl feeding is It is advisable not to escape. After reacting for 1.5 hours, the material was introduced into the reaction kettle E4 to continue chlorination, the reaction temperature was controlled at 90° C., and HCl was continued to react for 1 hour.
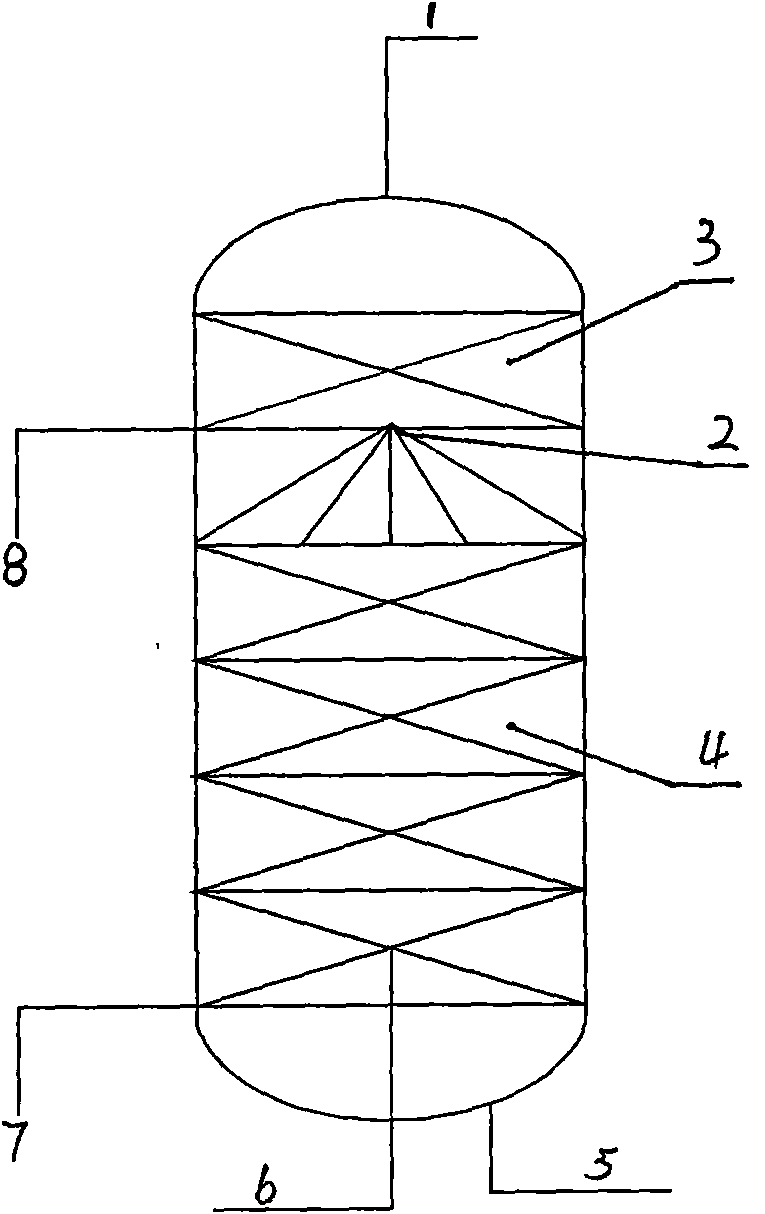
[0029] The material after the primary reaction is introduced into the spray reaction tower E8 equipped with corrugated mesh packing, the temperature inside the tower is controlled at 110°C, HCl is continuously introduced from E7 to the bottom of the spray reaction tower E8, and the pressure at the outlet of the HCl gas is adjusted to 0.3~ 0.4MPa, the glycerin is completely reacted, and the reaction ends when no distillate flows out o...
Embodiment 2
[0032] Embodiment 2: basically with embodiment 1, just reduced a reactor E4, glycerin concentration 95%, catalyzer is the propionic acid that accounts for the amount of glycerin substance 5%, is heated to 70 ℃, continues reaction 2 hours. The top of the spraying reaction tower can obtain 99.3% dichloropropanol, the conversion rate of glycerol is 100% in the reaction process, and the yield of dichloropropanol is 96.2%.
Embodiment 3
[0033] Embodiment 3: see figure 1 , 2 93% of the glycerol in the head tank E1 is passed into the reactor E3, and simultaneously the acetic acid catalyst accounting for 5% of the glycerin substance amount and the p-toluenesulfonic acid catalyst of 3% of the glycerol substance amount are passed into, heated to 65° C., and automatically Steel cylinder E2 continuously feeds HCl gas into E3, and the amount of HCl that is fed in should not escape. After reacting for 1 hour, the materials were introduced into the reaction kettle E4 to continue chlorination, the reaction temperature was controlled at 90° C., and HCl was continued to react for 1 hour.
[0034] The material after the primary reaction is introduced into the spray reaction tower E8 equipped with corrugated mesh packing, the temperature inside the tower is controlled at 110°C, HCl is continuously introduced from E7 to the bottom of the spray reaction tower E8, and the pressure at the outlet of the HCl gas is adjusted to 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com