Pre-filled recombinant human interferon injection
A technology of recombinant human interferon and injection, applied in the field of recombinant human interferon injection, can solve the problems of protein aggregation, affecting the titer of interferon, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
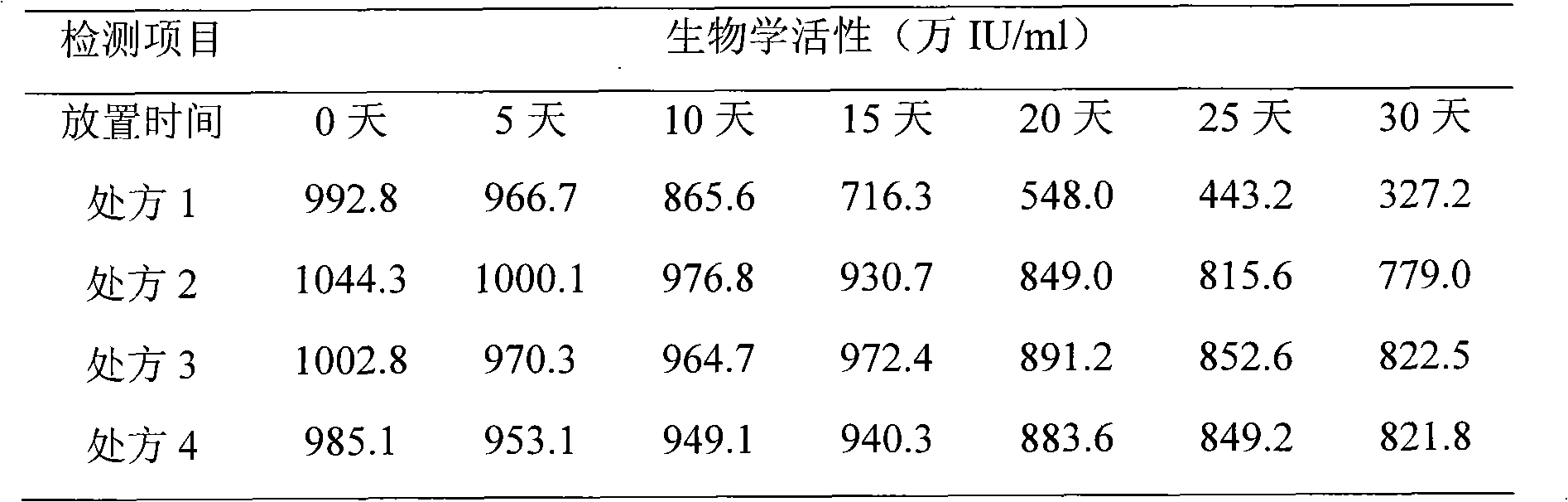
Embodiment 1
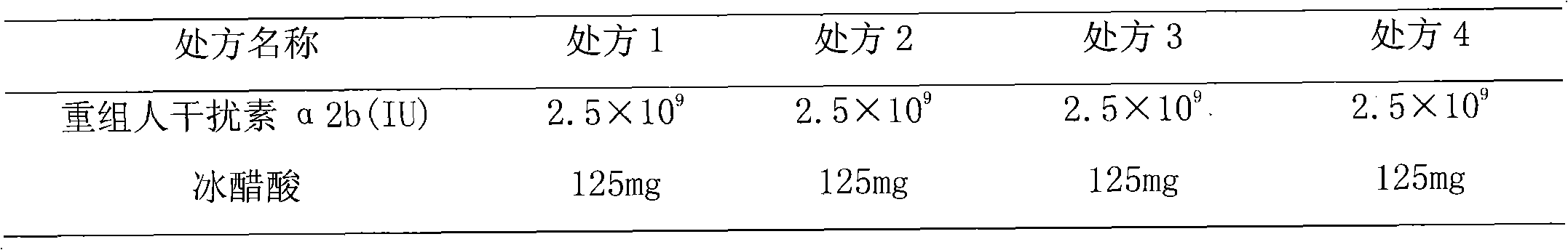
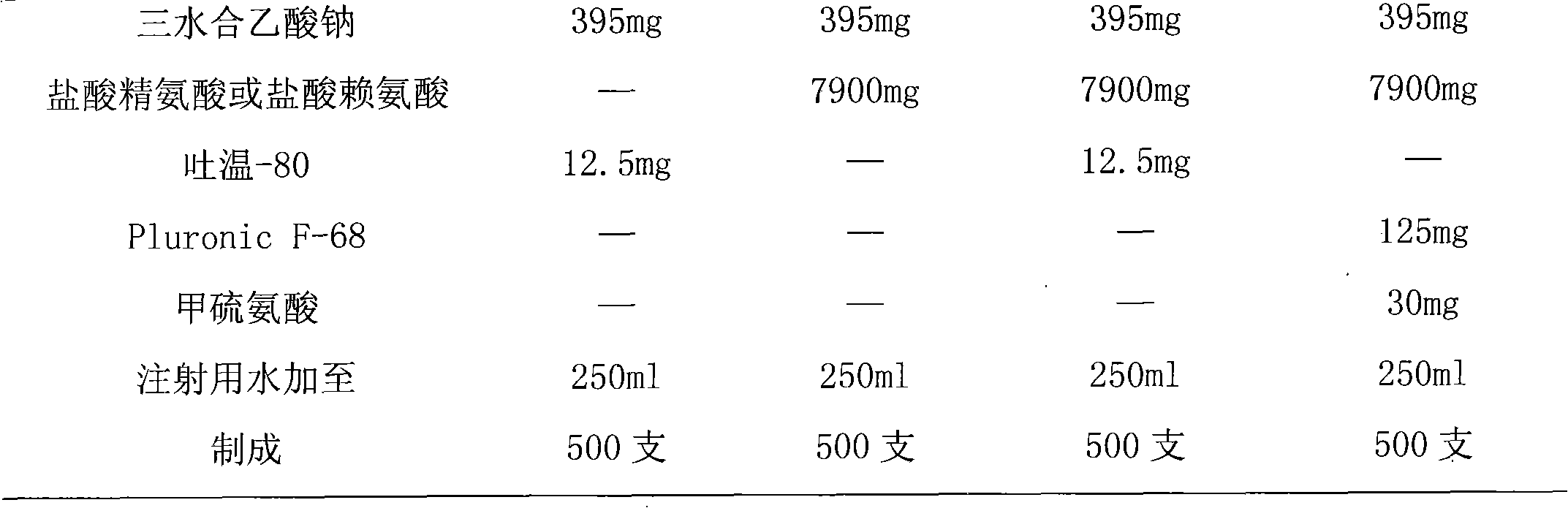
[0038] Prescription: recombinant human interferon α2b: 2.5×10 9 (IU), sodium acetate trihydrate 395mg, glacial acetic acid 125mg, arginine hydrochloride 7900mg, Tween-80 12.5mg, water for injection was added to 250mL;
[0039] Configuration method:
[0040] (1) Take the raw materials of the prescription amount, dissolve arginine hydrochloride, sodium acetate trihydrate, and glacial acetic acid with sterile pyrogen-free water for injection;
[0041] (2) Take Tween-80 and add it to the above-mentioned dissolved raw material solution;
[0042] (3) Add the recombinant human interferon α2b of the prescribed amount to the solution obtained in step (2), and mix well;
[0043] (4) Dilute water for injection to 250mL, pass through a microporous membrane with a pore size of 0.22μm, store in a refrigerator at 8±2°C, and take samples for sterility and heat source inspection;
[0044] (5) Subpackage the qualified injection in the step (4) into 1 mL vials, each containing 0.5 mL, to obta...
Embodiment 2
[0046]Prescription: recombinant human interferon α2b: 2.5×10 9 (IU), sodium acetate trihydrate 395mg, glacial acetic acid 125mg, arginine hydrochloride 7900mg, Tween-80 12.5mg, water for injection was added to 250mL;
[0047] Configuration method:
[0048] (1) Take the raw materials of the prescription amount, dissolve arginine hydrochloride, sodium acetate trihydrate, and glacial acetic acid with sterile pyrogen-free water for injection;
[0049] (2) Take Tween-80 and add it to the above-mentioned dissolved raw material solution;
[0050] (3) Add the recombinant human interferon α2b of the prescribed amount to the solution obtained in step (2), and mix well;
[0051] (4) Dilute water for injection to 250mL, pass through a microporous membrane with a pore size of 0.22μm, store in a refrigerator at 8±2°C, and take samples for sterility and heat source inspection;
[0052] (5) Divide the qualified injection in step (4) into prefilled syringes, and fill each syringe with 0.5 m...
Embodiment 3
[0054] Prescription: recombinant human interferon α2b: 1.5×10 9 (IU), sodium acetate trihydrate 237mg, glacial acetic acid 75mg, lysine hydrochloride 4740mg, Tween-80 12mg, water for injection was added to 150mL;
[0055] Configuration method:
[0056] (1) Take by weighing the prescription amount of raw materials, lysine hydrochloride, sodium acetate trihydrate, and glacial acetic acid are dissolved with sterile pyrogen-free water for injection;
[0057] (2) Take Tween-80 and add it to the above-mentioned dissolved raw material solution;
[0058] (3) Add the recombinant human interferon α2b of the prescribed amount to the solution obtained in step (2), and mix well;
[0059] (4) Dilute water for injection to 150mL, pass through a microporous membrane with a pore size of 0.22 μm, store in a refrigerator at 8±2°C, and take samples for sterility and heat source inspection;
[0060] (5) Subpackage the qualified injection in the step (4) into 1 mL vials, each containing 0.3 mL, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com