Stabilizer for recombinant alpha-interferon liquid
A liquid stabilizer, interferon technology, applied in the direction of interferon, cytokine/lymphokine/interferon, specific peptide, etc., can solve the problem of the high price of human albumin, the increase of the cost of interferon products, the carrying of human albumin virus and other problems, to achieve the effect of obvious protection effect, lightening economic burden and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
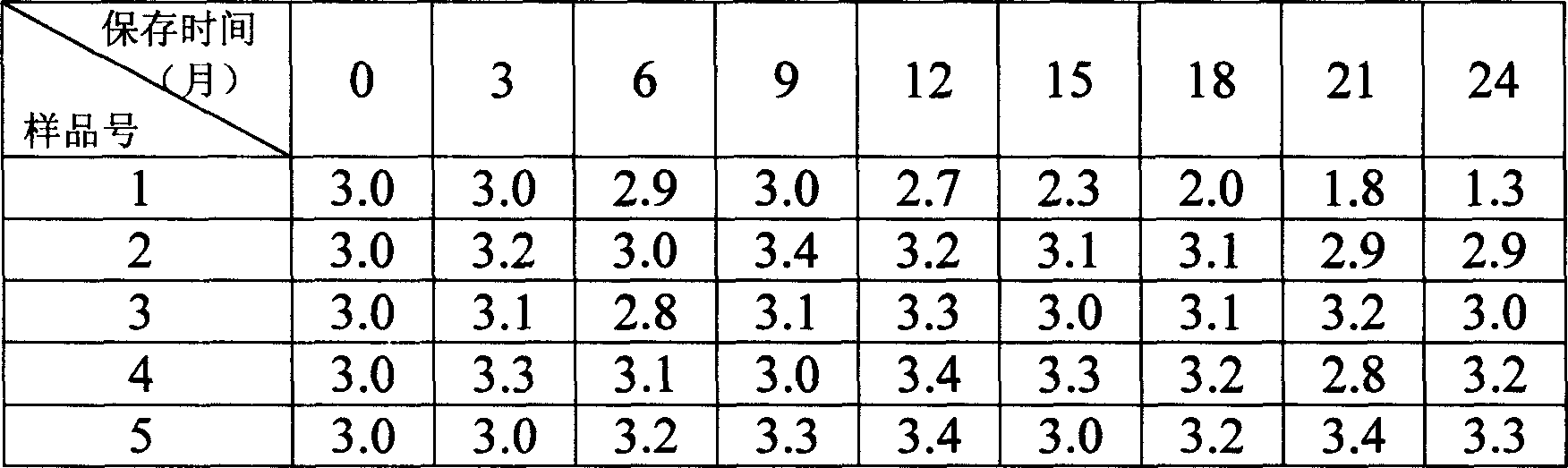
[0010] Take gelatin, sucrose, polyethylene glycol, and glycine, and prepare them with 0.85% normal saline, wherein the gelatin, sucrose, polyethylene glycol, and normal saline solutions are sterilized by autoclaving, and the glycine solution is sterilized by filtration to prepare 2- No. 5 stabilizer, each concentration is as follows, No. 1 stabilizer is human serum albumin, as a control group, each sample contains recombinant α-type interferon 1-10 million IU / ml, fully mixed, sterilized and filtered, and packaged The amount is 1ml / branch.
[0011] The following is the stability of the interferon injection with the above-mentioned stabilizer and human albumin as a stabilizer under different conditions: (wherein the interferon titer is within 80-150% of the labeled amount, which means that the interferon activity has not decreased. )
[0012] No. 1 stabilizer: 1% human serum albumin, control group.
[0013] No. 2 stabilizer: 0.4% gelatin + 3% sucrose
[0014] No. 3 stabilizer...
Embodiment 2
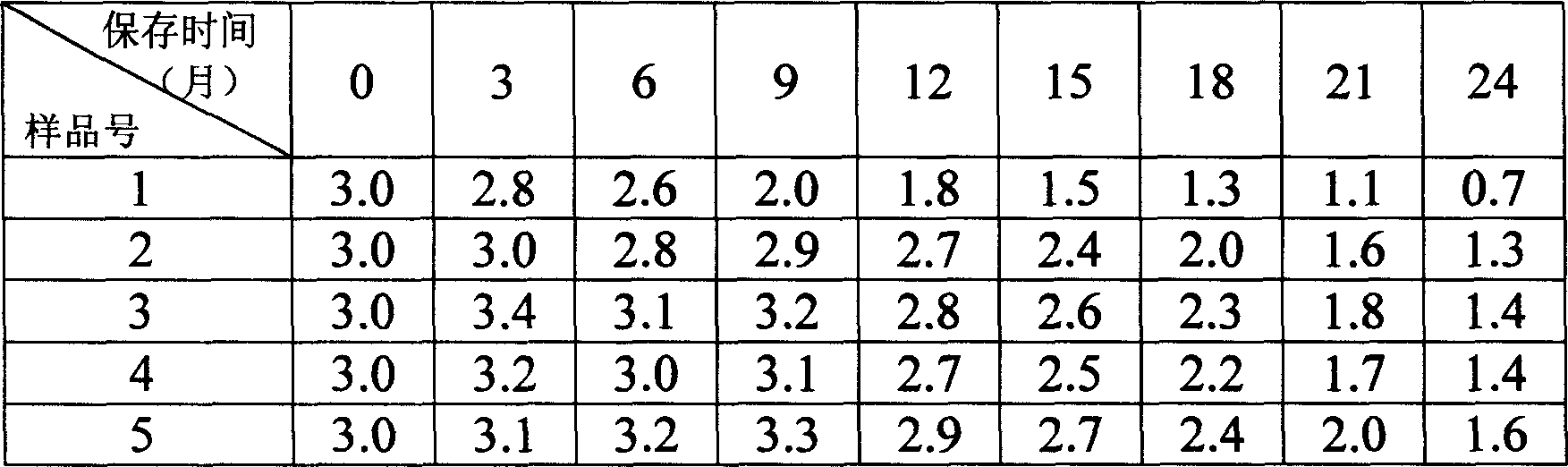
[0025] Experimental results on gelatin and different disaccharide compositions.
[0026] No. 2-1: 0.5% gelatin + 2% sucrose
[0027] Size 2-2: 0.5% gelatin + 2% lactose
[0028] No. 2-3: 0.5% gelatin + 2% trehalose
[0029] Repeat above-mentioned process step, result is as follows:
[0030] Table 4: Stability of accelerated test interferon at 40°C (containing interferon 5 million IU / ml)
[0031]
Embodiment 3
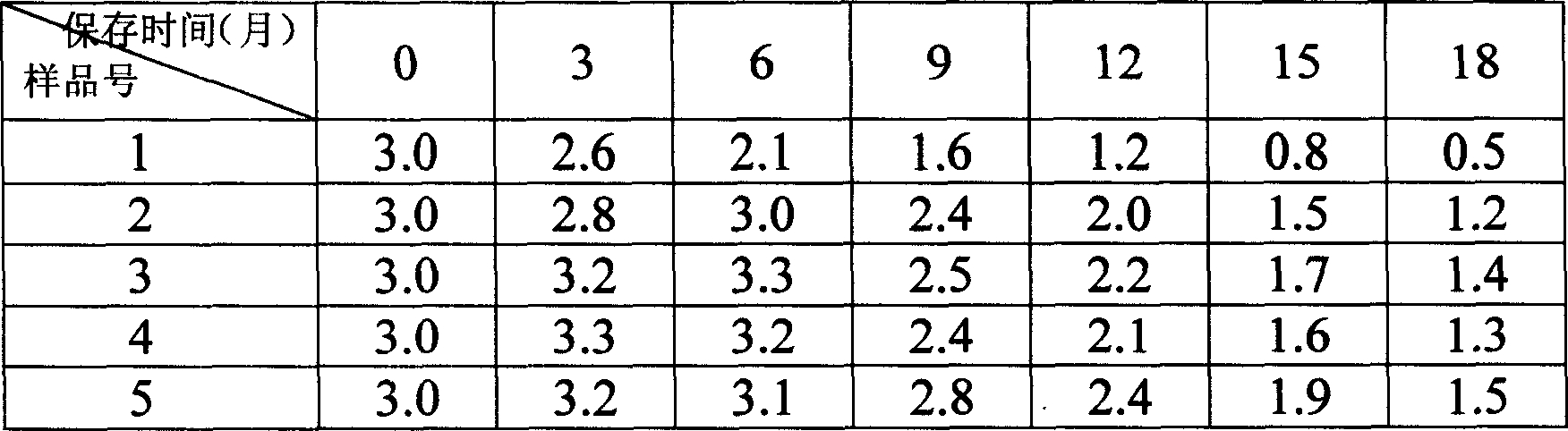
[0033] Experimental results of gelatin and disaccharide compositions with different amino acids.
[0034] No. 4-1: 0.4% gelatin + 2% lactose + 0.5% glycine
[0035] No. 4-2: 0.4% gelatin + 2% lactose + 0.5% arginine
[0036] No. 4-3: 0.4% gelatin + 2% lactose + 0.5% lysine
[0037] Table 5: Stability of accelerated test interferon at 40°C (containing interferon 3 million IU / ml)
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com