Methods for isolating long fragment rna from fixed samples
A long-segment, tissue-sample technology that is used in the determination of chemotherapy-based regimens and can address issues such as failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0209] Example 1: Extraction steps of long fragment RNA
[0210] I. Tissue Preparation
[0211] Paraffin blocks containing 10 micron sections of FFPE tissue were mounted on glass slides without coverslips using standard protocols. For deparaffinization and nuclear fast red (NFR) staining, slides were processed as follows:
[0212] Slides were washed twice with xylene for 5 minutes each, followed by ethanol ("EtoH") washes. The slides were stained with NFR using standard protocol.
[0213] The area of interest (eg, tumor tissue or stroma) is dissected by hand or with a laser capture microdissector (depending on the size of the resection).
[0214] II. RNA extraction
[0215] An extraction solution containing Tris / HCL, EDTA, SDS and water was prepared. Add tumor tissue and proteinase K to the extraction solution in the centrifuge tube. The sample is then heated at an appropriate temperature for an appropriate time in order to obtain a maximum yield of long fragment RNA, ...
Embodiment 2
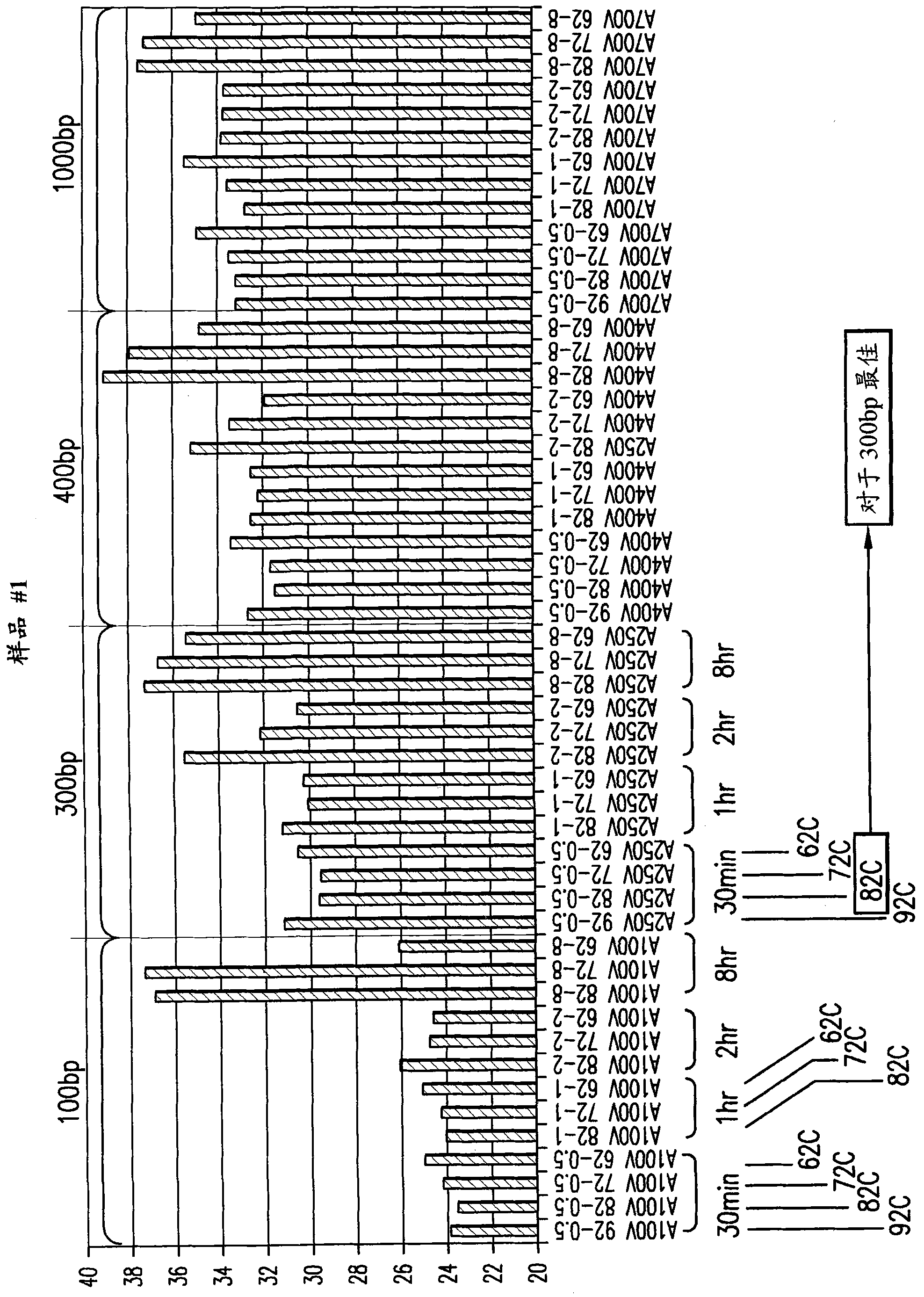
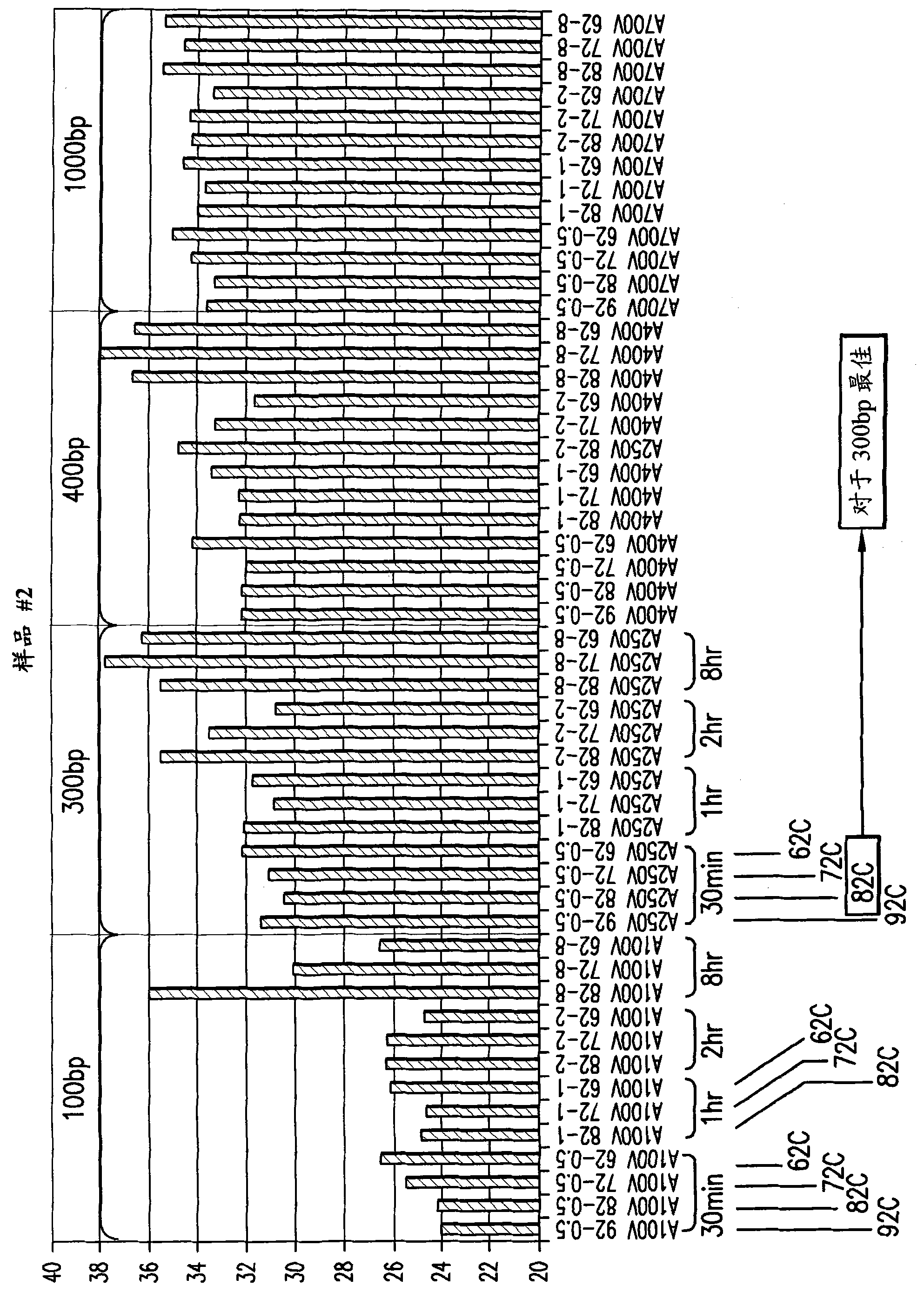
[0218] Example 2: Method for Determining the Length Distribution of Isolated RNA
[0219] To determine the relative amounts of various RNA fragment lengths isolated from FFPE tissues, the following strategy was used. RNA isolated from FFPE samples using the present invention and other known extraction methods is converted to cDNA using oligo dT primers. This means that only mRNA fragments containing the 3'-oligo-A tail will be extended and converted into cDNA, providing a starting point for measuring fragment length. PCR amplification of β-actin mRNA was used to represent the total mRNA population. Primers were chosen to amplify a segment of approximately 100-120 bp representing the β-actin gene at positions 100, 300, 400 and 1000 bp from the 3'-end of the mRNA (Figure 2). Using this strategy, any differences in the length-dependent efficacy of the amplification were minimized, rather than actually trying to amplify 100, 300, 400 and 1000 bp fragments. Therefore, the Ct of ...
Embodiment 3
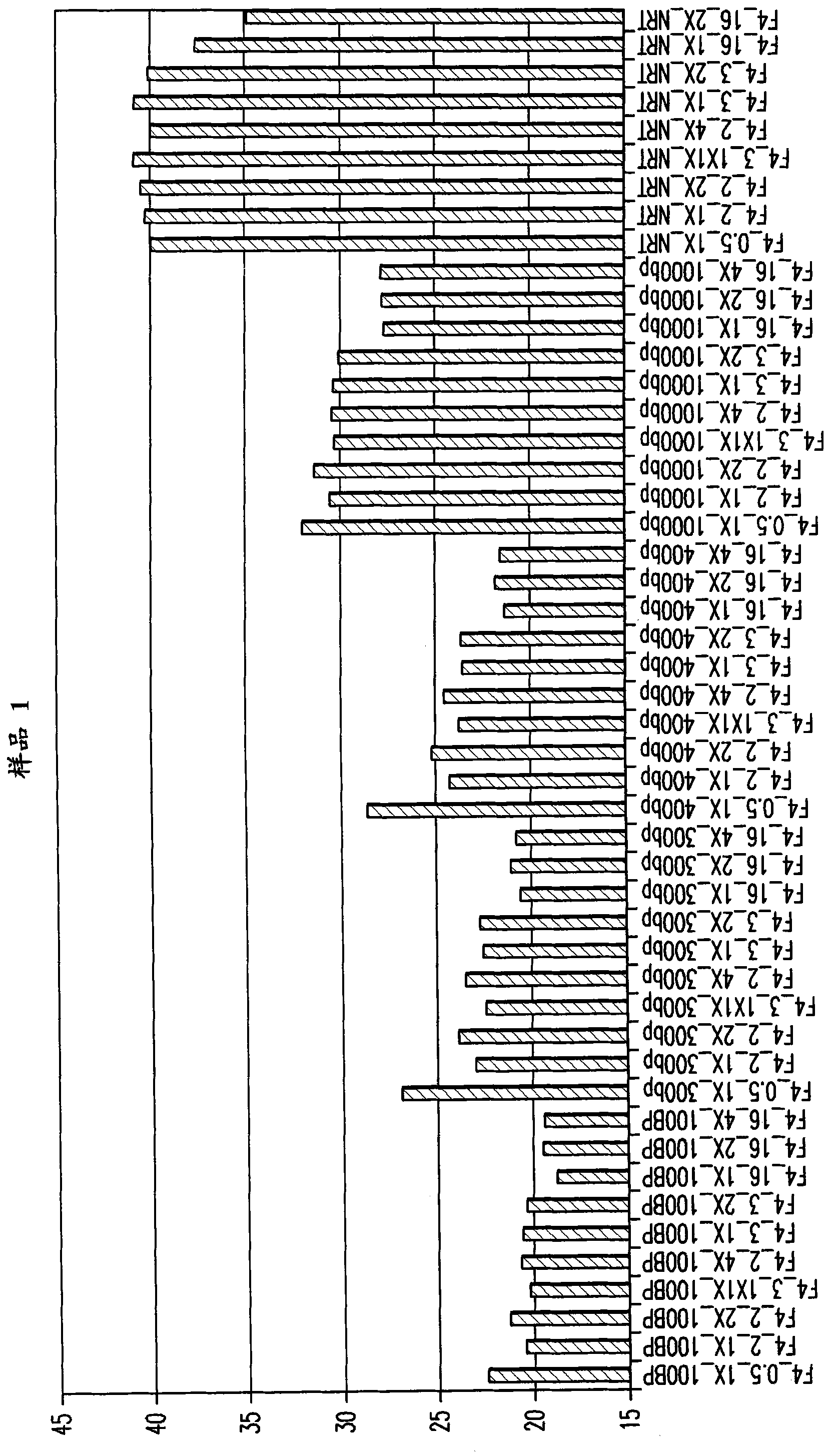
[0222] Example 3: Effect of Proteinase K
[0223] This example demonstrates the effect of proteinase K concentration on RNA yield and DNA contamination. Proteinase K concentration was varied over a 4-fold range (5-20 μg, 1X-4X as indicated in the graph) at 50°C with incubation times of 0.5, 2, 3 and 16 hours. like Figure 4As shown, 1X (5 μg) of proteinase K gave a greater amount of approximately 2-fold (1 Ct) RNA yield, however more importantly, amounts of proteinase K greater than 1X gave visible higher DNA contamination (2 -3Ct cycle). This experiment also demonstrates the effect of incubation time on the amount of DNA extracted, with a 16 hour incubation time being 3 to 7 Ct cycles better than shorter incubation times. DNA in the extracts was detected by performing PCR without first performing a reverse transcription reaction to convert the RNA to cDNA ("no reverse transcription or NRT control"). This way, only PCR amplification of the co-extracted DNA occurs, if it is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com