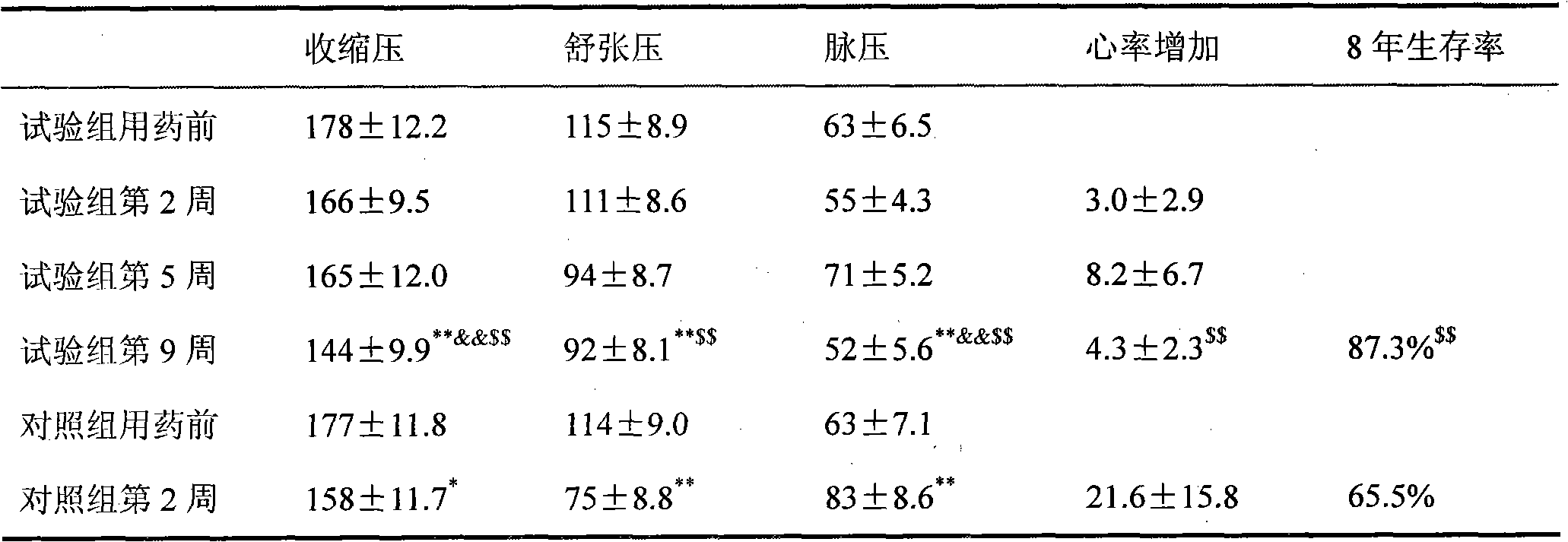
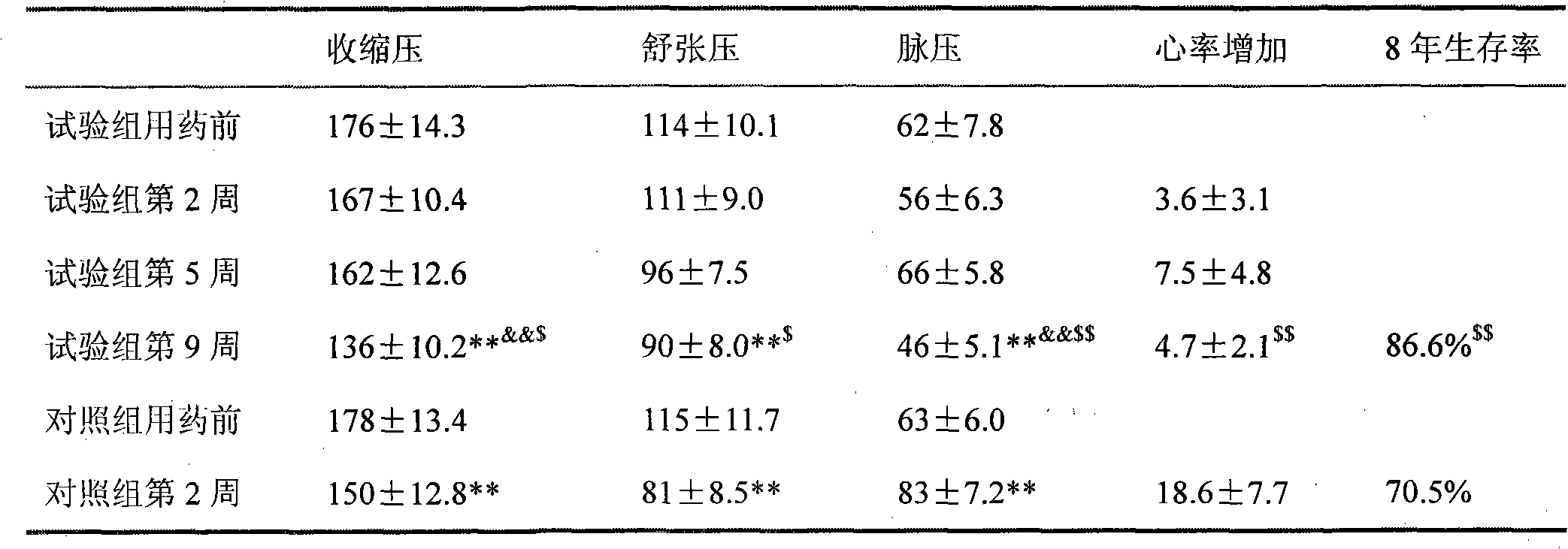
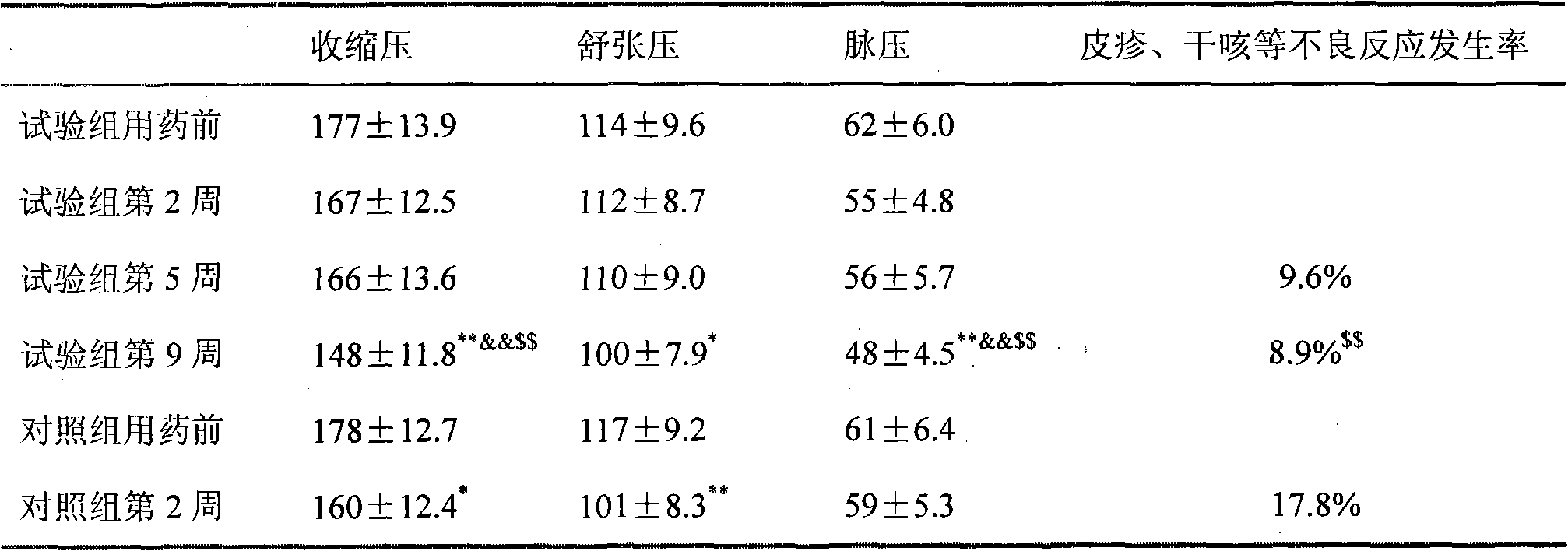
Medicine combination containing isosorbide mononitrate for treating high blood pressure
A technology of isosorbide dinitrate and high blood pressure, which is applied in the field of medicine, can solve problems such as increased pulse pressure, and achieve the effects of reducing systolic blood pressure and pulse pressure difference, reducing arterial pressure, and lowering blood pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Isosorbide Mononitrate Nitrendipine Extended Release Tablets
[0020] Prescription A
[0021] Isosorbide Mononitrate 15g
[0022] Starch 50g
[0023] L--HPC 20g
[0024] 10% starch slurry appropriate amount
[0026] Prescription B
[0027] Nitrendipine 15g
[0028] HPMC--4M 20g
[0029] HPMC--15M 30g
[0030] Microcrystalline Cellulose 20g
[0031]PVP 8g
[0032] Micronized silica gel 35g
[0033] 8% PVPk30 absolute ethanol solution appropriate amount
[0035] Preparation Process:
[0036] Pass the isosorbide mononitrate, starch, and L-HPC in prescription A through a 100-mesh sieve, mix well, add 10% starch slurry to granulate, dry below 50°C, granulate with a 18-mesh sieve, and add stearic acid Magnesium mix well; pass nitrendipine, HPMC--4M, HPMC--15M, microcrystalline cellulose, PVP, and micropowder silica gel in prescription B respectively through a 100-mesh sieve, mix well, add an appropria...
Embodiment 2
[0038] Isosorbide Mononitrate Captopril Tablets
[0039] Isosorbide Mononitrate 10g
[0040] Captopril 50g
[0041] Starch 60g
[0042] Microcrystalline Cellulose 100g
[0043] L-HPC 30g
[0044] 10% starch slurry appropriate amount
[0045] Magnesium Stearate 2g
[0046] Preparation Process:
[0047] Pass the isosorbide mononitrate, captopril, starch, microcrystalline cellulose and L-HPC in the prescription through a 100-mesh sieve respectively, mix well, add 10% starch slurry to granulate in an appropriate amount, dry below 50°C, and dry for 18 Mesh sieve for granulation, add prescription amount of magnesium stearate, mix evenly, and compress into tablets.
Embodiment 3
[0049] Isosorbide Mononitrate Nifedipine Extended Release Tablets
[0050] Prescription A
[0051] Isosorbide Mononitrate 20g
[0052] Starch 100g
[0053] Sodium carboxymethyl starch 10g
[0054] 10% starch slurry appropriate amount
[0055] Magnesium Stearate 1g
[0056] Prescription B
[0057] Nifedipine 15g
[0058] Cetyl Alcohol 10g
[0059] Glyceryl monostearate 20g
[0060] Microcrystalline Cellulose 40g
[0061] 8% PVPk30 absolute ethanol solution appropriate amount
[0062] Magnesium Stearate 2g
[0063] Preparation Process:
[0064] Pass the isosorbide mononitrate, starch, and sodium carboxymethyl starch in prescription A through a 100-mesh sieve, mix well, add 10% starch slurry to granulate, dry below 50°C, granulate with a 18-mesh sieve, add hard Magnesium stearate and mix well; pass the nifedipine, cetyl alcohol, glyceryl monostearate and microcrystalline cellulose in prescription B respectively through a 100-mesh sieve, mix well, add 8% PVPk30 absolut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com