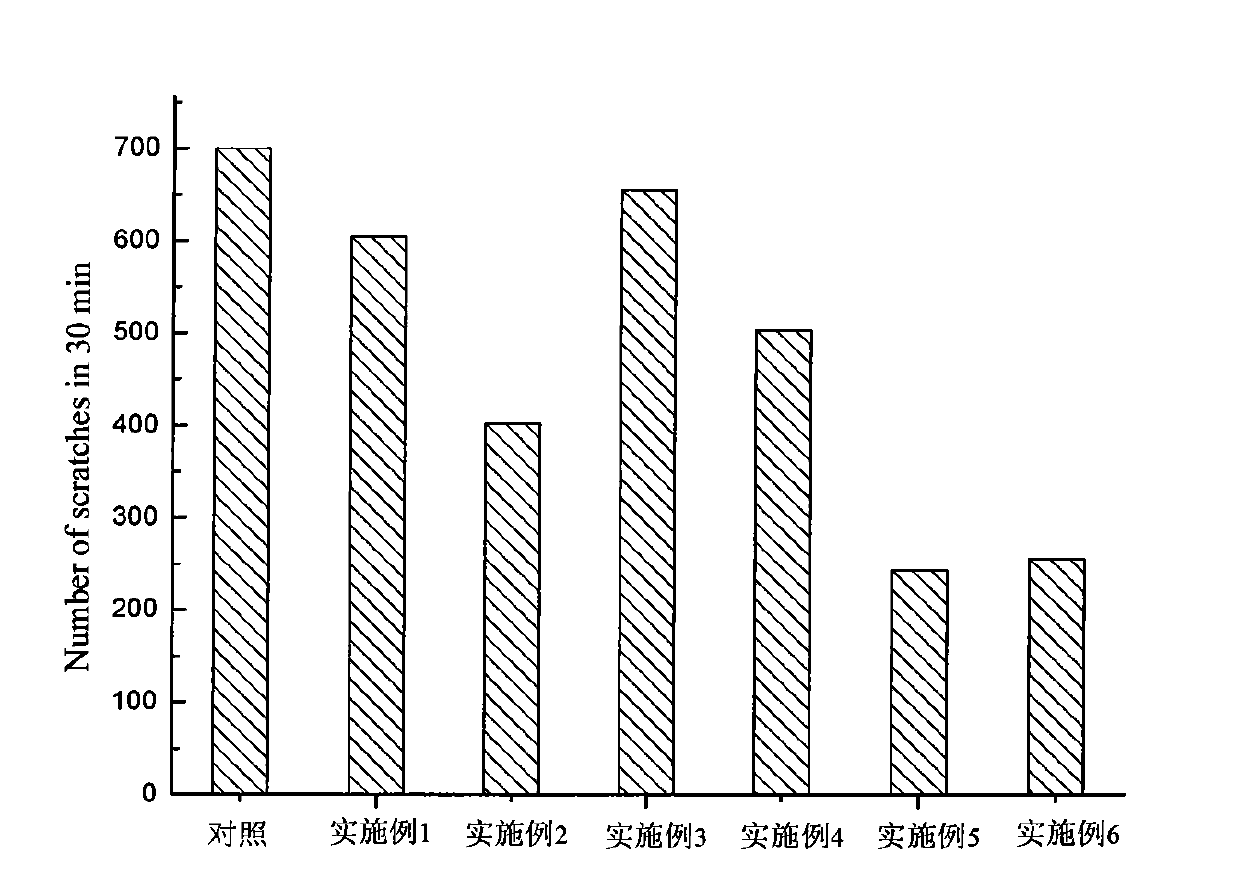
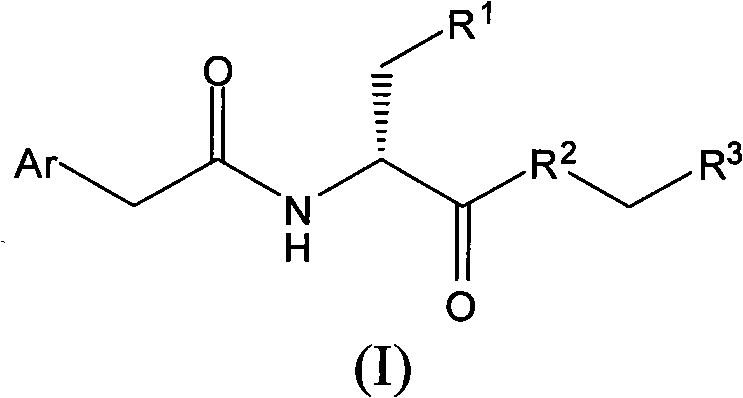
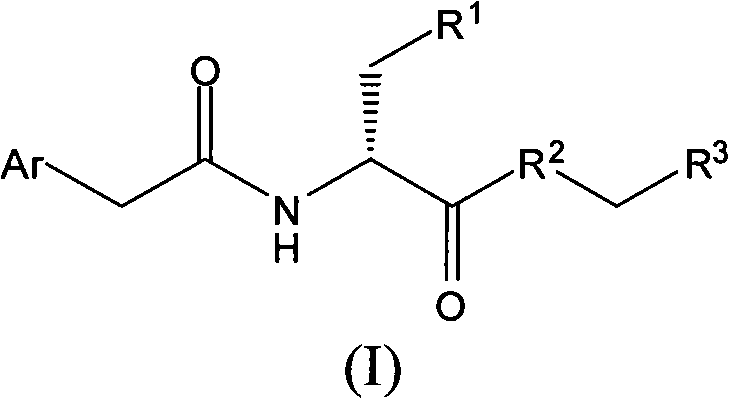
Compound having anti-itching activity
A compound and antioxidant activity technology, applied in the field of pharmaceutical compounds, can solve problems such as large molecular structure, and achieve the effect of moderate molecular weight and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038] synthetic route:
[0039]
[0040]
[0041] Step A: Compound 1 (Ethyl 2-(1H-imidazol-1-yl)acetate)
[0042] Add imidazole (8.17g, 120mmol), TEA (17.34mL, 120mmol), THF (50mL), ethyl chloroacetate (10.56mL, 100mmol) sequentially, reflux and stir for 10h, suction filter after the reaction, and separate the filtrate by column chromatography 12.1 g of yellow liquid was obtained, and the yield was 78.6% (based on ethyl chloroacetate), 1 H NMR (CDCl 3 -d 6 )δ: 7.49(s, 1H), 7.08(s, 1H), 6.94(s, 1H), 4.68(s, 2H), 4.23(q, 2H), 1.25(t, 3H).
[0043] Step B: Compound 2 (2-(1H-imidazol-1-yl)ethanol)
[0044] Add compound 1 (7.71g, 50mmol), methanol (90mL), LiCl (4.24g, 100mmol) in sequence, slowly add NaBH 4 (4.73g, 125mmol), reacted at 50°C for 8h, separated by column chromatography to obtain 4.31g light yellow oil, and the yield was 76.8% (based on compound 1), 1 H NMR (CDCl 3 -d 6 )δ: 7.47 (s, 1H), 7.02 (s, 1H), 6.91 (s, 1H), 4.01 (t, 2H), 3.65 (m, 2H)....
Embodiment 2
[0055] Example 2 ((R)-2-(2-(4-fluorophenyl)acetamido)-3-phenylpropanoic acid-2-(1H-imidazol-1-yl)ethyl ester)
[0056]
[0057] The operation steps are the same as in Example 1, except that in step D, 4-fluorophenylacetic acid is used instead of 4-nitrophenylacetic acid. The final product is a pale yellow oil, 1 H NMR (CDCl 3 -d 6 )δ: 6.84~7.43 (m, 12H), 4.76 (m, 1H), 4.29 (t, 2H), 4.12 (t, 2H), 3.49 (s, 2H), 2.94 (dd, 2H).
Embodiment 3
[0058] Example 3 ((R)-2-(2-(4-methylphenyl)acetamido)-3-phenylpropanoic acid-2-(1H-imidazol-1-yl)ethyl ester)
[0059]
[0060] The operation steps are the same as in Example 1, and in step D, 4-methylphenylacetic acid is used instead of 4-nitrophenylacetic acid. The final product is a pale yellow oil, 1 H NMR (CDCl 3 -d 6 )δ: 6.81~7.35(m, 12H), 4.75(m, 1H), 4.21(t, 2H), 4.08(t, 2H), 3.49(s, 2H), 2.95(dd, 2H), 2.34(s , 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com