Application of phospholipid scramblase 1 in preparing anti-hepatitis B virus infective medicament
A technology of phospholipid crawling enzymes and uses, which is applied in antiviral agents, pharmaceutical formulas, medical preparations containing active ingredients, etc., to achieve important social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
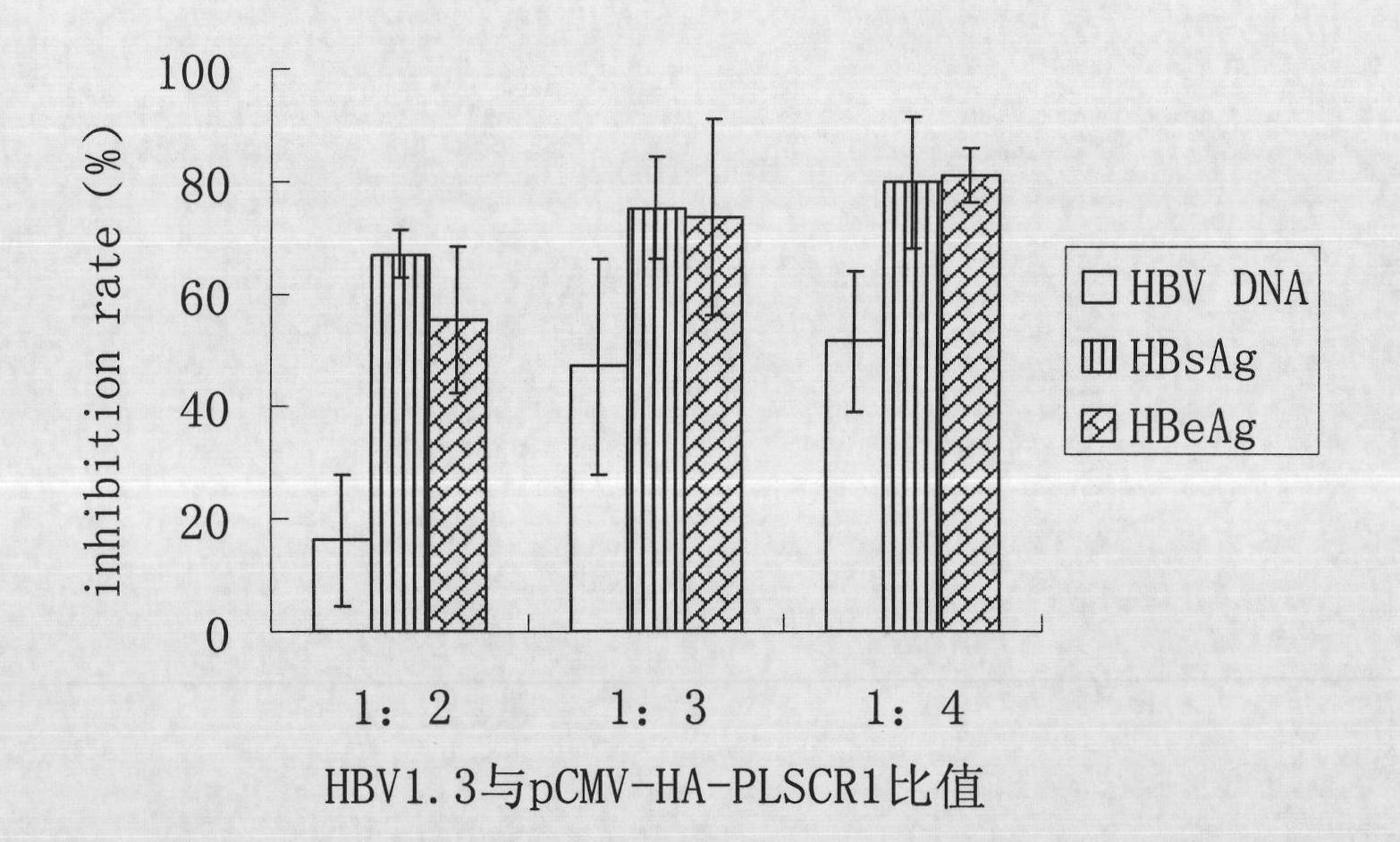
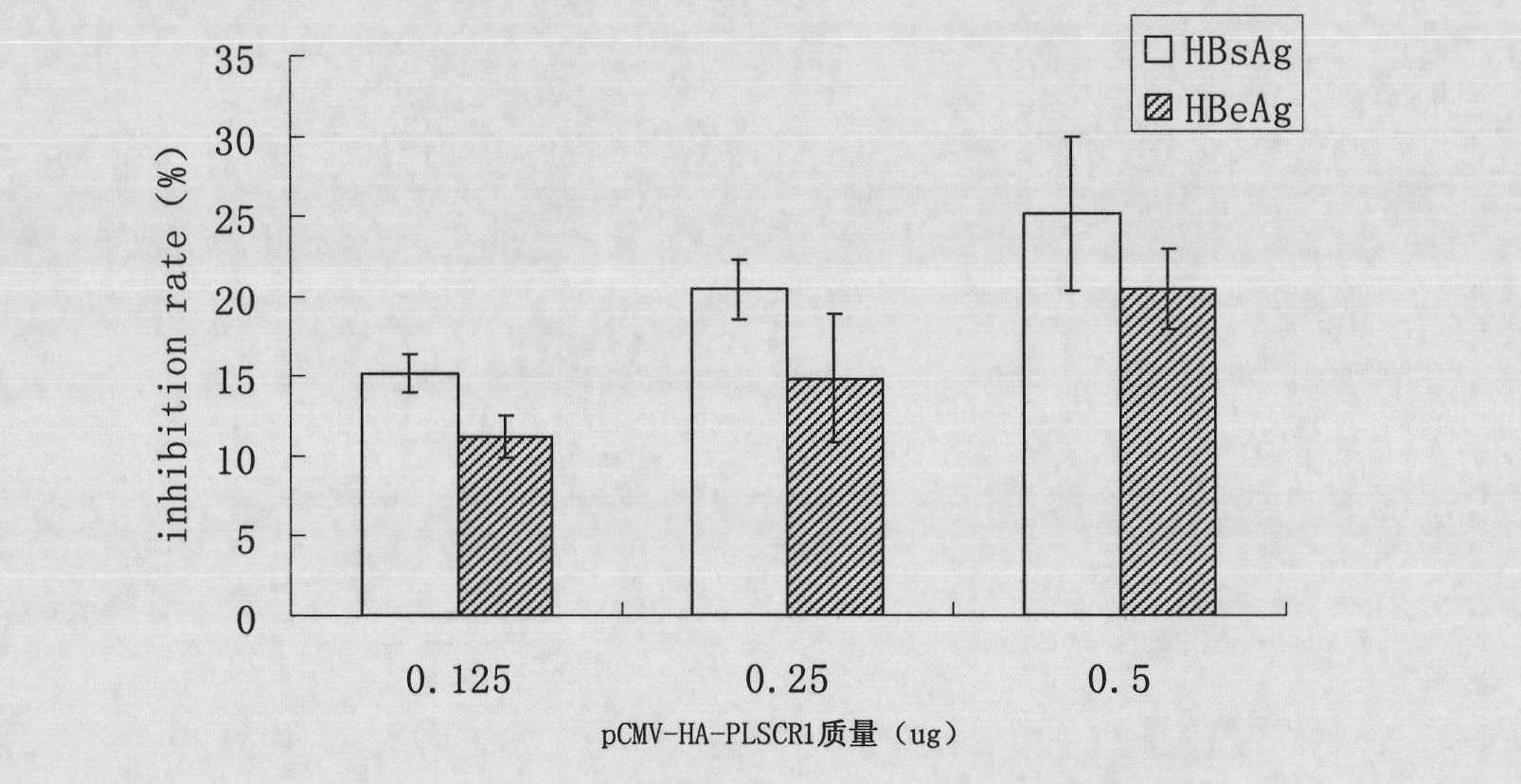
[0034] Example 1 Inhibition of Intracellular Expression of PLSCR1 on HBV Replication
[0035] Materials and Methods
[0036] 1. Cell culture
[0037] The cells used are the liver cancer cell line HepG2 cell line and the HepG2.2.15 cell line transfected with HBV DNA. HepG2.2.15 cells are derived from HepG2 cells, contain integrated HBV DNA, and can continuously and stably secrete Dane's granules, HBsAg, HBV DNA, etc. into the culture medium during cell culture. HepG2 cells were cultured with DMEM cell culture medium containing 10% fetal bovine serum (FBS, Gibco), and HepG2.2.15 cells were cultured with MEM cell culture medium containing 10% fetal bovine serum, 380 μg / ml G418 (Promega).
[0038] 2. Plasmid
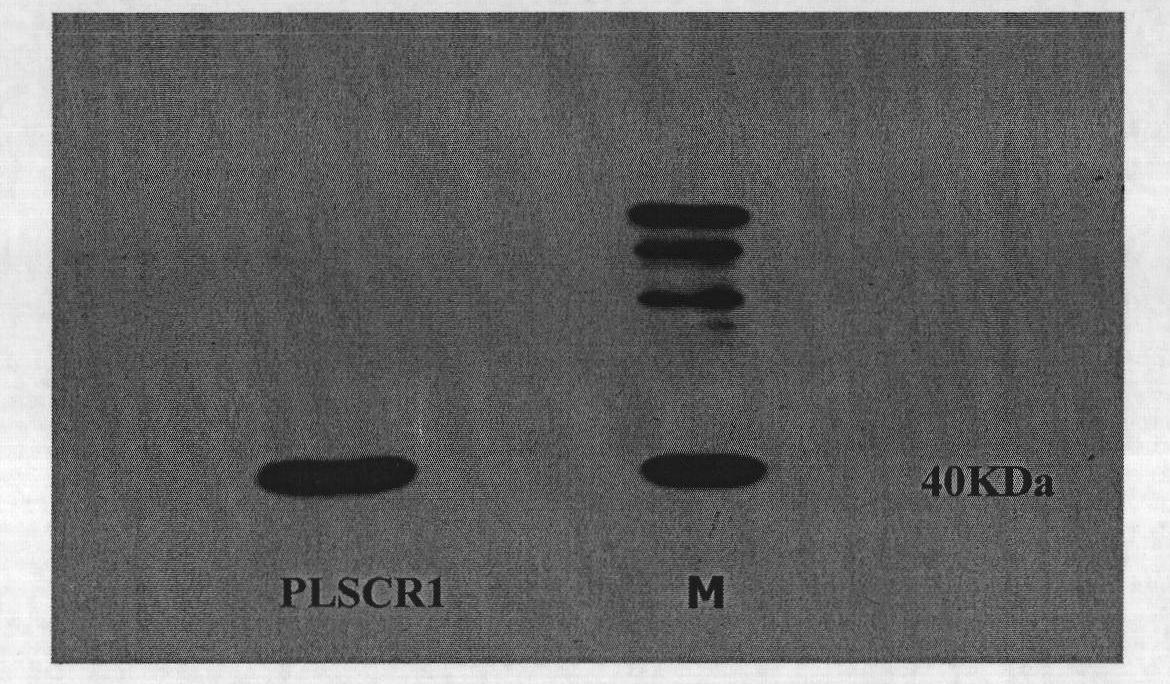
[0039] The pCMV-HA-PLSCR1 plasmid was constructed by our laboratory, and primers were designed according to the PLSCR1 sequence (NM_021105.2) published by NCBI to amplify the PLSCR1 CDS region, which was inserted into the pCMV-HA plasmid by cloning, screened by positive c...
Embodiment 2
[0059] Example 2: Inhibition of HBV replication by adding exogenous recombinant GST-PLSCR1 protein
[0060] Materials and Methods
[0061] 1. Construction of pGEX-4T-1-PLSCR1 plasmid
[0062] pGEX-4T-1-PLSCR1 was constructed in our laboratory, and primers were designed according to the PLSCR1 sequence (NM_021105.2) published by NCBI to amplify the PLSCR1 CDS region, which was inserted into the pGEX-4T-1 plasmid by cloning, and positively cloned Screening, sequencing, and BLAST comparison results showed that the constructed pGEX-4T-1-PLSCR1 was completely correct.
[0063] 2. Induced expression of GST-PLSCR1 fusion protein
[0064] Inoculate Escherichia coli DH5α transformed with the vector pGEX-4T-1-PLSCR1 expressing GST fusion protein into LB medium, shake overnight at 37°C, then inoculate into fresh LB medium according to 2% inoculum size, and culture at 30°C After 5 hours, IPTG was added to a final concentration of 0.1 mmol / L-1 mmol / L, and induction culture was continued...
Embodiment 3
[0090] Example 3: Inhibition of HBV replication by adding exogenous recombinant His-PLSCR1 fusion protein
[0091] Materials and Methods
[0092] 1. Construction of Pet28a-PLSCR1 plasmid
[0093] Pet28a-PLSCR1 was constructed in our laboratory. Primers were designed according to the PLSCR1 sequence (NM_021105.2) published by NCBI to amplify the CDS region of PLSCR1, which was inserted into the Pet28a plasmid by cloning. The positive clones were screened, sequenced, and compared by BLAST The results showed that the constructed Pet28a-PLSCR1 was completely correct.
[0094] 2. Induced expression and purification of His-PLSCR1 fusion protein
[0095] Inoculate the Escherichia coli containing the recombinant expression plasmid on an agar plate, pick a single colony from a fresh agar plate, inoculate 2ml LB medium, add appropriate antibiotics (final concentration 100mg / L) in a 20ml culture tube, shake Cultivate overnight on the bed, dilute the bacteria overnight at a ratio of 1:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com