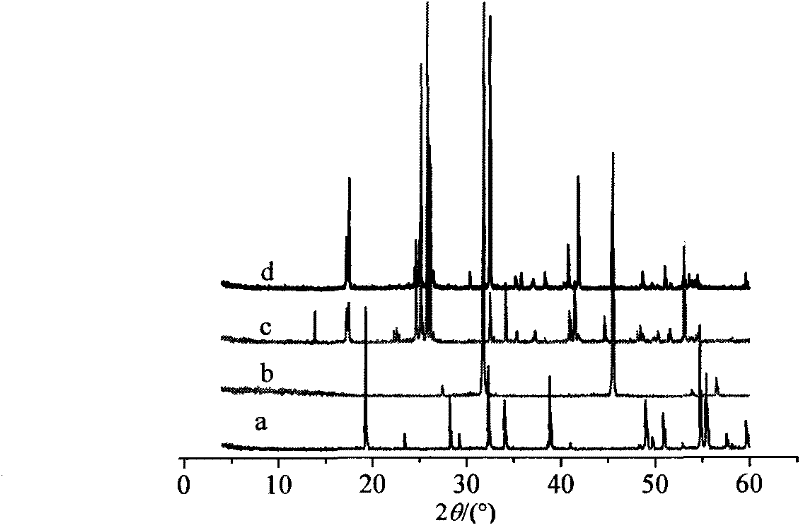
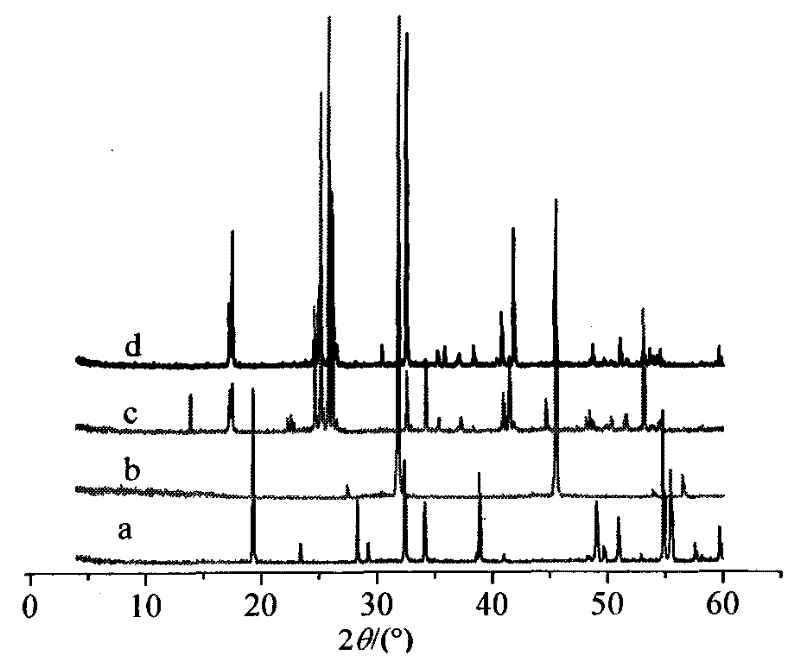
Method for producing hydrogen chloride and sodium hydrogen sulfate crystals by taking rock salt as raw material
A technology of sodium bisulfate and sodium sulfate, applied in the direction of sulfate/bisulfate preparation, chlorine/hydrogen chloride, etc., can solve the problems of unfavorable transfer and packaging, easy hardening and water absorption of salt, complex operation process, etc., to prevent crystallization The effect of tower condition, avoiding dissolution loss, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: In a 50L glass-lined reactor with a stirred tank and a thermometer, add 6.00kg of 96% sulfuric acid first, then add 6.20kg of sodium chloride mixture with a mass percentage of sodium sulfate of 0.01%, and simultaneously start hydrogen chloride absorption or storage system; keep stirring at a constant temperature of 100°C, and add 12.39kg of 96% sulfuric acid, and keep warm for 30 minutes after adding acid; introduce the reaction solution into a crystallization tower containing 3.82kg of water, and first Stirring crystallization 5min, then static crystallization 5min, finally stirring crystallization 20min; Suction filtration and washing with saturated sodium bisulfate solution give sodium bisulfate crystal 14.52kg, analyze wherein sodium bisulfate crystal mass percentage composition is 96.98%, free acid (according to Sulfuric acid meter) mass percentage composition is 1.98%, and chloride ion mass percentage composition is 0.10%; Filtrate is mixed with the sulf...
Embodiment 2
[0023] Embodiment 2: Add 6.00kg 90% sulfuric acid earlier in the 50L glass-lined reaction kettle with stirring tank and thermometer, then add 6.20kg sodium sulfate mass percentage composition and be the sodium chloride mixture of 0.1%, open hydrogen chloride absorption simultaneously or Storage system; Stir continuously at a constant temperature of 110°C, and add 17.07kg of 90% sulfuric acid, keep warm for 25 minutes after adding acid; introduce the reaction solution into a crystallization tower containing 2.86kg of water, under the condition of condensed water temperature of 10°C, Stir and crystallize first 10min, then leave standstill to crystallize 10min, finally stir and crystallize 35min; Suction filtration and wash with saturated sodium bisulfate solution to obtain sodium bisulfate crystal 14.55kg, analyze wherein sodium bisulfate crystal mass percentage composition is 97.56%, free acid ( In terms of sulfuric acid), the mass percentage is 1.93%, and the chloride ion mass ...
Embodiment 3
[0024] Embodiment 3: Add 6.00kg90% sulfuric acid to a 50L glass-lined reactor with a stirring tank and a thermometer, then add 6.20kg of sodium chloride mixture with a mass percentage of sodium sulfate of 0.5%, and simultaneously start hydrogen chloride absorption or storage System; keep stirring at a constant temperature of 120°C, and add 22.77kg of 90% sulfuric acid, and keep warm for 20 minutes after adding acid; introduce the reaction solution into a crystallization tower containing 1.91kg of water, and first Stirring and crystallization 20min, then static crystallization 10min, finally stirring crystallization 45min; Suction filtration and washing with saturated sodium bisulfate solution to obtain sodium bisulfate crystal 14.51kg, analysis wherein sodium bisulfate crystal mass percentage composition is 97.47%, free acid (according to Sulfuric acid meter) mass percentage composition is 1.85%, and chloride ion mass percentage composition is 0.23%; Filtrate is mixed with the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com