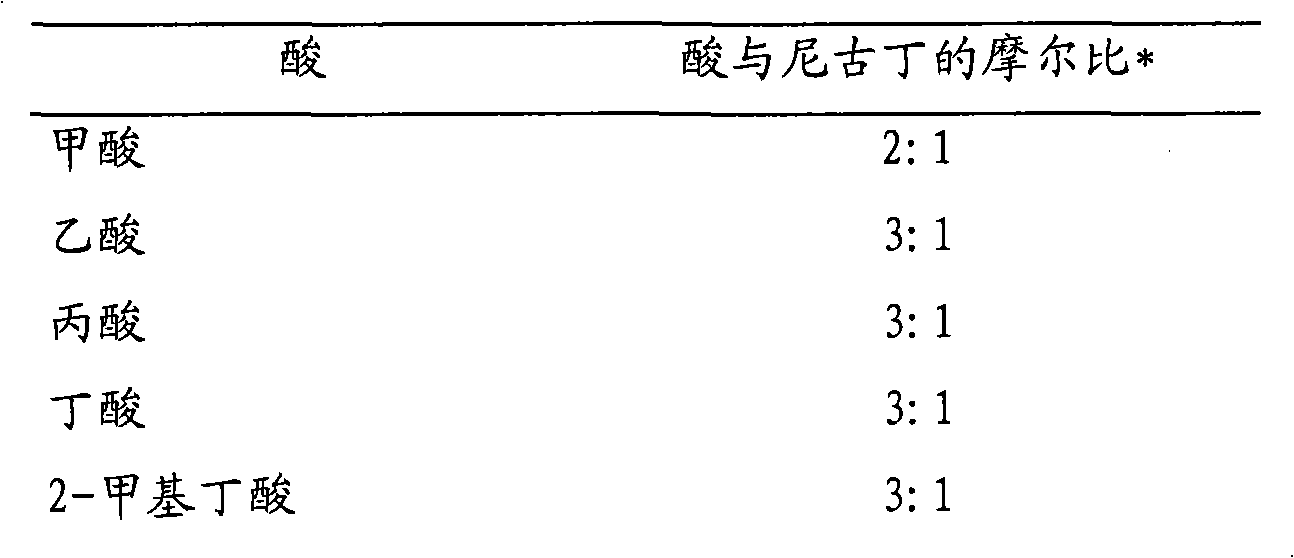
Oral nicotine formulation buffered with amino acid
A technology for oral preparation and nicotine, applied in the field of nicotine-containing pharmaceutical preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0160] Example 1 tablet
[0161] 275mg tablet with 2mg nicotine
[0162] Element
Amount in the composition (mg)
Nicotine bitartrate dihydrate
6.1
[0163] Element
Amount in the composition (mg)
L-Arginine
17.9
210.9
11.0
Cross-linked polyvinylpyrrolidone
11.0
flavoring agent
9.9
1.6
1.1
5.5
[0164] making process :
[0165] Mix the above ingredients. The blend is then compressed into tablets by direct compression according to methods known in the art.
example 2
[0166] Example 2 Fusion film
[0167] This is a tablet designed to melt in the mouth when the melting substance adheres to the oral mucosa where nicotine is deposited to enter the tissues.
[0168] 400mg mouth-melting tablet with 2mg nicotine
[0169] Element
Relative amount in composition (% w / w)
Nicotine bitartrate dihydrate
1.5
cocoa powder
35.0
41.6
L-Arginine
4.5
10.4
Titanium dioxide
2.7
1.0
0.4
[0170] Element
Relative amount in composition (% w / w)
0.2
flavoring agent
2.7
[0171] making process :
[0172] The preparation of this formulation is carried out at room temperature. A portion of the fat component (ie vegetable oil) is melted. The solid ingredients (ie nicotine salt, cocoa powder, buffer, mannitol, titanium dioxide, sweetener ...
example 3
[0174] Instance 3A mouth spray
[0175] Nicotine mouth spray containing 14.3 mg nicotine per ml and pH 9.0
[0176] Element
[0177] Element
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com