Atom economic preparation method for laminated composite metal hydroxide
A hydroxide and layered composite technology, applied in the direction of oxide/hydroxide preparation, etc., can solve the problems of unavoidable by-product formation and washing process, structure cannot be restored, etc., to achieve the effect of protecting the environment and saving water resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Step A: Mg(OH) 2 and Al(OH) 3 by Mg 2+ / Al 3+ The molar ratio is mixed in a ratio of 3:1, and 10 g of the mixture is placed in 90 g of deionized water, and then added to the reactor;
[0030] Step B: Add 1.27g of ammonium bicarbonate and 10ml of ammonia water into the reactor, raise the temperature to 100°C, react for 2 hours, centrifuge the obtained product and directly dry it at 70°C for 8 hours to obtain the molecular formula Mg 6 Al 2 (OH) 12 CO 3 4H 2 LDHs product of O, namely MgAl-CO 3 -LDHs.
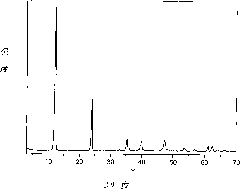
[0031] The crystal structure of the sample was characterized by XRD-6000 X-ray powder diffractometer from Shimadzu Corporation, Japan. figure 1 It is the XRD spectrogram of the sample obtained in Example 1. As can be seen from the figure, the 003, 006 and 009 crystal plane diffraction peaks reflecting the layered structure appear at 2θ=11.7°, 23.4° and 34.5° respectively. The peak shape of the diffraction peak is sharp, the baseline is low and flat, and no impurit...
Embodiment 2
[0034] Step A: Zn(OH) 2 , Mg(OH) 2 and Al(OH) 3 Press Zn 2+ : Mg 2+ : Al 3+ The molar ratio is mixed in a ratio of 1:3:2, and 15g of the mixture is placed in 80g of deionized water, and then added to the reactor;
[0035] Step B: Add 1.27g of ammonium bicarbonate and 20ml of ammonia water into the reactor, raise the temperature to 200°C, react for 1 hour, centrifuge the obtained product and directly dry it at 70°C for 8 hours to obtain the molecular formula ZnMg 3 Al 2 (OH) 12 CO 3 4H 2 O's LDHs products.
Embodiment 3
[0037] Step A: Ca(OH) 2 and Al(OH) 3 Press Ca 2+ : Al 3+ The molar ratio is mixed in a ratio of 3:1, and 20 g of the mixture is placed in 80 g of deionized water, and then added to the reactor;
[0038] Step B: Add 1.27g of ammonium bicarbonate and 30ml of ammonia water into the reactor, raise the temperature to 200°C, react for 1 hour, centrifuge the obtained product, and directly dry at 70°C for 8 hours to obtain the molecular formula Ca 4 Al 2 (OH) 12 CO 3 4H 2 O's LDHs products.
[0039] The elemental content of the samples was analyzed by a Shimadzu ICPS-7500 elemental analyzer. Elemental analysis shows that the sample prepared in Example 3 shows that Ca in the product: Al=3: 1, and does not contain Na in the product + and other hetero ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com