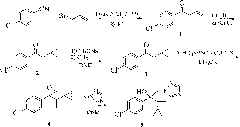
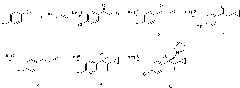Method for synthesizing chiral cyproconazole
A synthesis method and cyproconazole technology are applied in the field of synthesis technology of pesticide cyproconazole, can solve problems such as consumption increase, and achieve the effects of high production cost, improved unit efficacy and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1) 1-(4-chlorophenyl)-2-cyclopropyl-1-propene A
[0051]Dissolve 1-(4-chlorophenyl)-2-cyclopropyl-1-propanone (20.8g, 100mmol) in pre-dried toluene, keep the temperature at -20°C, and add the pre-prepared In the toluene solution of methylenetriphenylphosphorous ylide (41.5g, 150mmol, 1mol / L), after the dropwise addition was completed, the temperature was slowly raised to room temperature, stirred at room temperature for 6 hours, and the raw material 1-(4-chlorobenzene When the content of -2-cyclopropyl-1-propanone is less than 1%, the reaction ends, and 0.6mol of water is added to the reaction system to quench the reaction. Distilled under reduced pressure to obtain 19.5 g of 1-(4-chlorophenyl)-2-cyclopropyl-1-propene with a yield of 95%.
[0052] 2) Synthesis of epoxidized intermediate B
[0053] 1-(4-Chlorophenyl)-2-cyclopropyl-1-propene A (20.6g, 100mmol) and catalytic amount of titanium tetraisopropoxide (5×10 -4 mol) was dissolved in DMF, keeping the temperature...
Embodiment 2
[0057] 1) Synthesis of 1-(4-chlorophenyl)-2-cyclopropyl-1-propene A
[0058] Dissolve 1-(4-chlorophenyl)-2-cyclopropyl-1-propanone (20.8g, 100mmol) in pre-dried dioxane, keep the temperature at -10°C, add dropwise to the system In the prepared dioxane solution of methylenetriphenylphosphorous ylide (55.3g, 200mmol, 1mol / L), after the dropwise addition was completed, the temperature was slowly raised to room temperature, stirred at room temperature for 8 hours, and the raw material 1 was analyzed by gas chromatography. The content of -(4-chlorophenyl)-2-cyclopropyl-1-propanone is less than 1%. After the reaction is completed, 1 mol of water is added to the reaction system to quench the reaction. Distilled under reduced pressure to obtain 19.2 g of 1-(4-chlorophenyl)-2-cyclopropyl-1-propene, with a yield of 93.1%.
[0059] 2) Synthesis of epoxidized intermediate B
[0060] 1-(4-Chlorophenyl)-2-cyclopropyl-1-propene A (20.6g, 100mmol) and a catalytic amount of salen-Mn (1×10 -...
Embodiment 3
[0064] 1) Synthesis of 1-(4-chlorophenyl)-2-cyclopropyl-1-propene A
[0065] Dissolve 1-(4-chlorophenyl)-2-cyclopropyl-1-propanone (20.8g, 100mmol) in pre-dried THF, keep the temperature at 0°C, and add the pre-prepared In the THF solution of methyltriphenylphosphorus ylide (82.8g, 300mmol, 1mol / L), after the dropwise addition was completed, the temperature was slowly raised to room temperature, stirred at room temperature for 4 hours, and the raw material 1-(4-chlorophenyl The content of )-2-cyclopropyl-1-propanone is less than 1%, and 1.2 mol of water is added to the reaction system to quench the reaction after the reaction is completed. Distilled under reduced pressure to obtain 20 g of 1-(4-chlorophenyl)-2-cyclopropyl-1-propene A with a yield of 97.2%.
[0066] 2) Synthesis of epoxidized intermediate B
[0067] 1-(4-chlorophenyl)-2-cyclopropyl-1-propene A (20.6g, 100mmol), catalytic amount of titanium trichloride (5×10 -4 mol) and 2,3-naphthyridine ligand (5×10 -4 mol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com