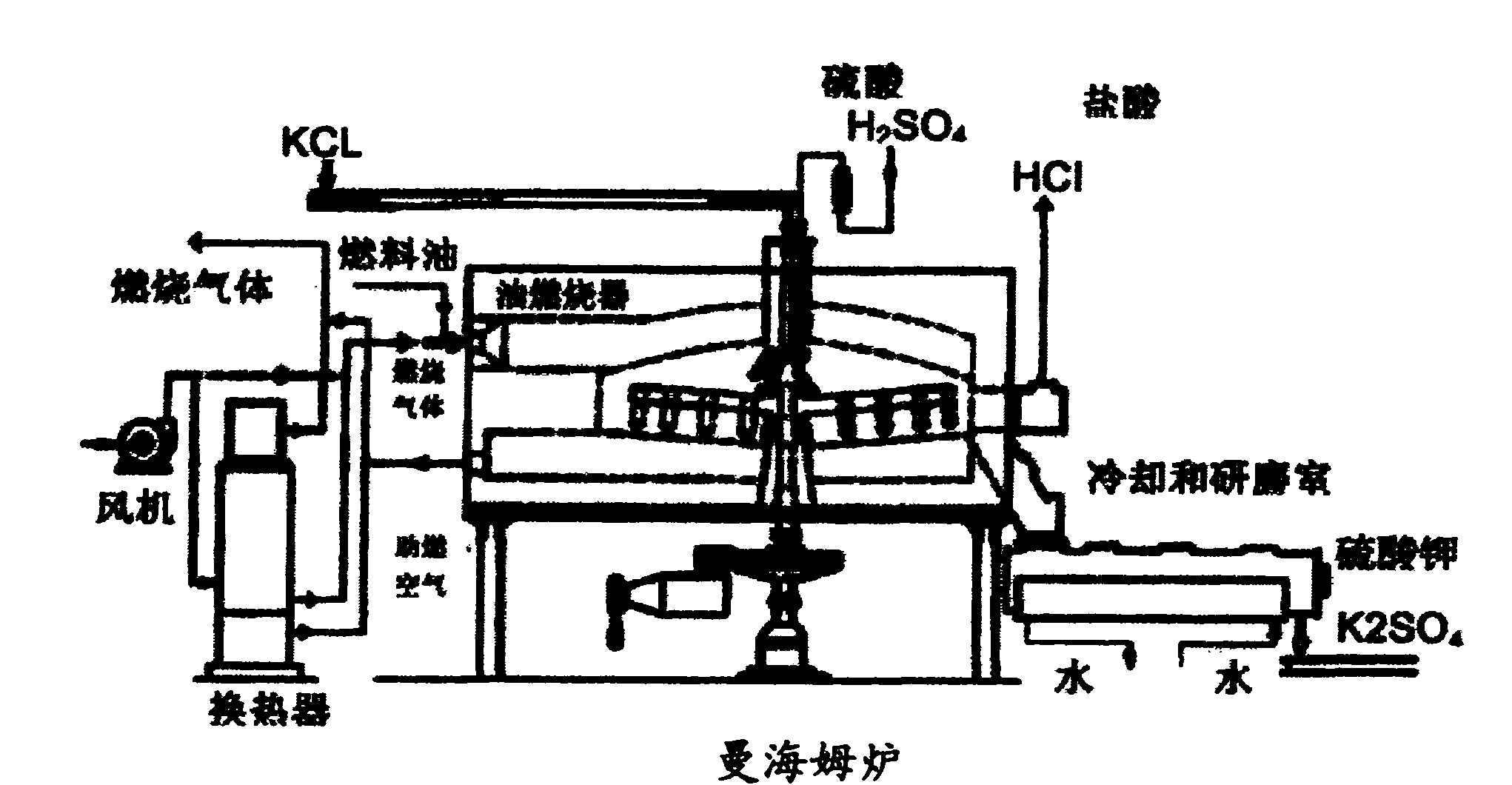
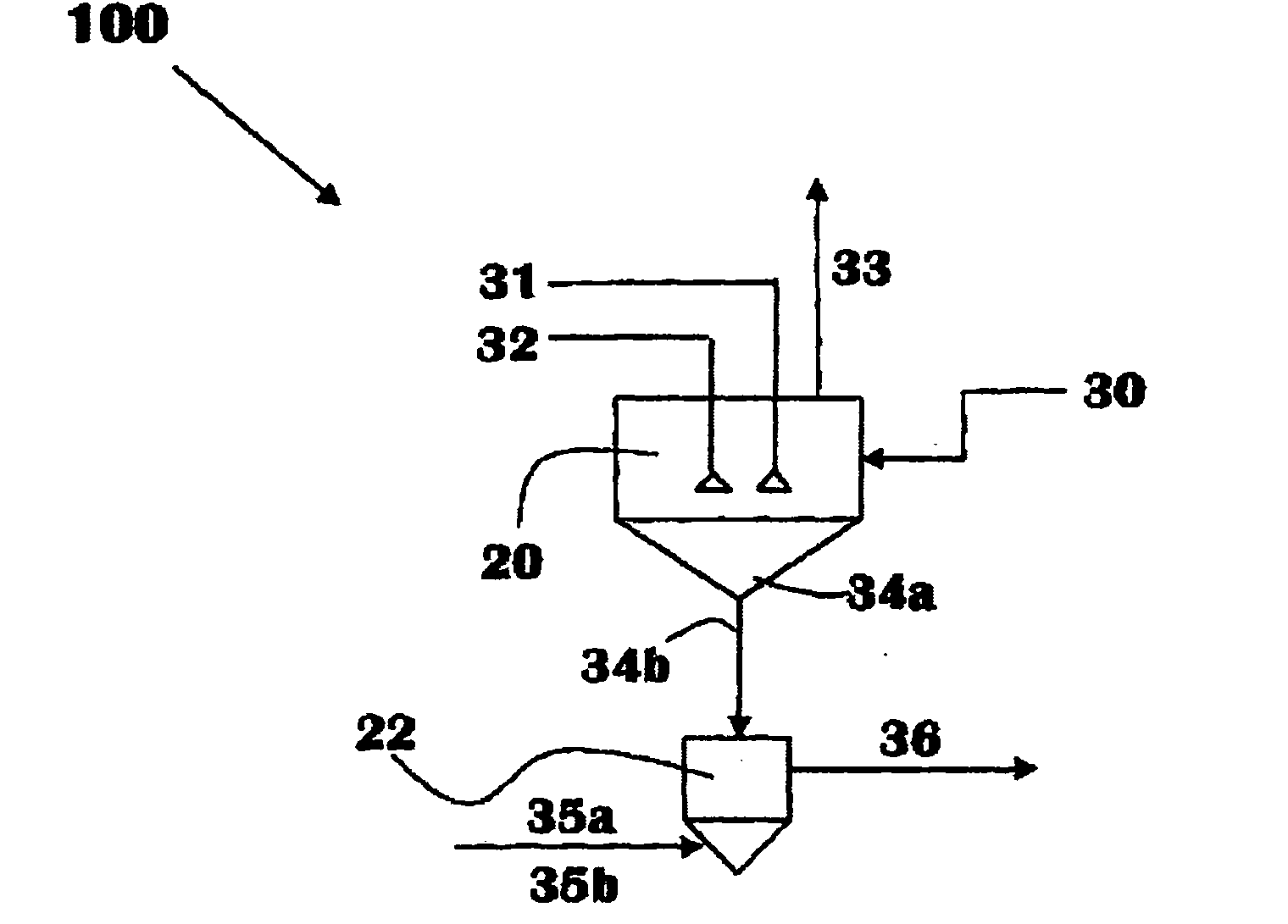
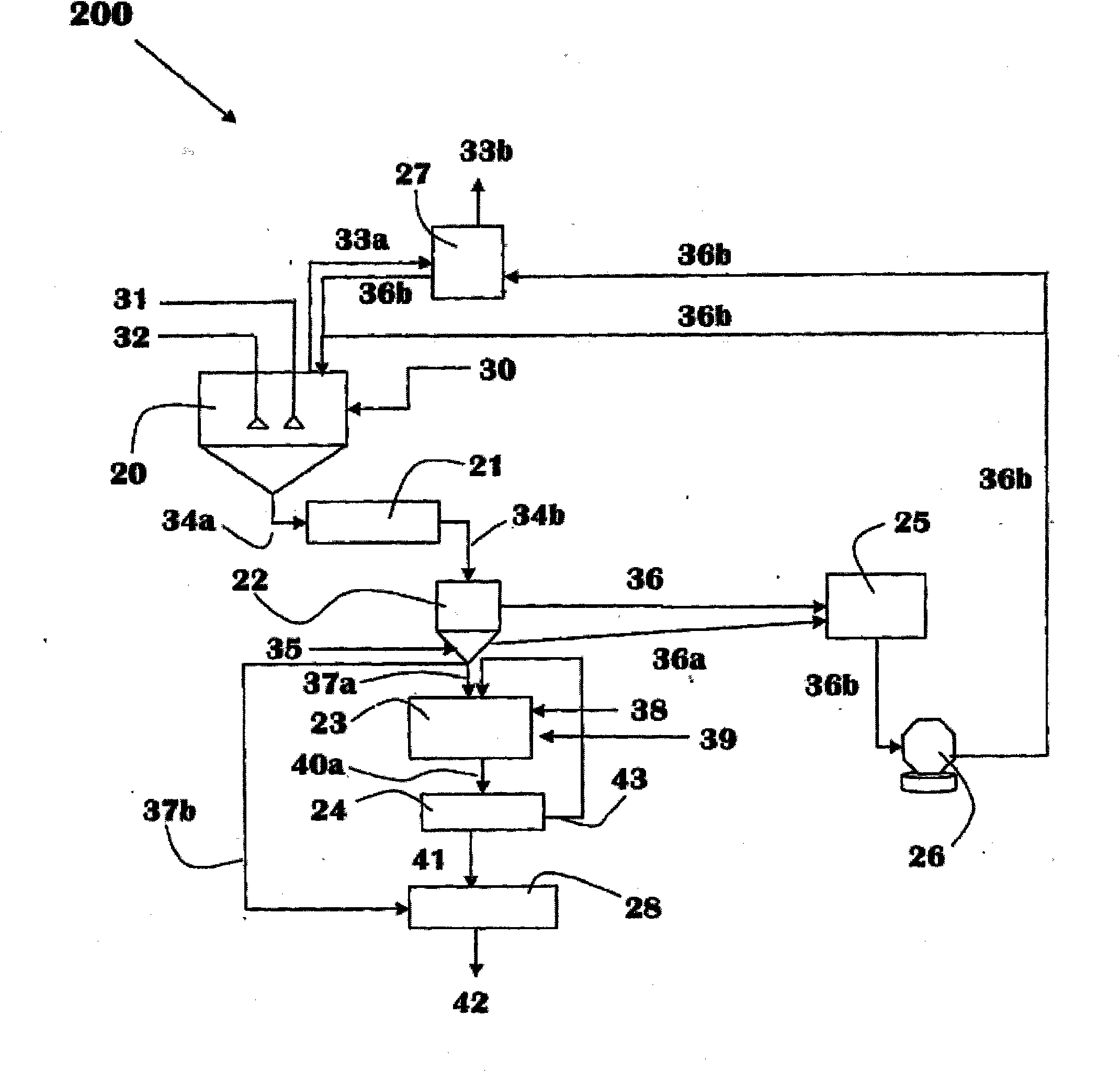
Chemical process to produce hydrogen chloride and chloride-free compound potassium sulfate fertilizers or other metal sulfates
A technology of metal sulfate and sulfate, applied in ammonium sulfate, chemical instruments and methods, sulfate preparations, etc., can solve problems such as low energy efficiency and inability to produce double salts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] By adding 200g of K 2 SO 4 , 200g of H 2 SO 4 and 600g H 2 O was mixed for 3 hours to prepare an aqueous liquid, typically the supernatant remaining after sulfate precipitation. 548 g of KCl were then dissolved in this aqueous liquid ("supernatant"). 673 g of fuming sulfuric acid (31% free SO 3 ) into the container containing this solution. The HCl discharged from the reaction mixture was dissolved in 520 g of water; the total weight of the aqueous HCl solution thus obtained was 786 g. This HCl solution was found to contain 32% HCl and 0.5% H 2 SO 4 . Then, the sulfate-containing solution obtained from the reaction mixture was cooled to 35° C., whereby the sulfate-containing salt was precipitated. Crystals were separated from the supernatant by filtration, then dried at -105°C for 2 hours. The weight of the dried crystals was 340 g (44.7% yield). The resulting salt contained 34.2% K, compared to pure K 3 H(SO 4 ) 2 The theoretical value is 37.8%, the salt...
Embodiment 2
[0115] Using 40g of H 2 O 100 g of the dried sulphate product from the previous example were further washed to remove any residual H 2 SO 4 . The solid was filtered under vacuum then dried at -105°C for 2 hours. The wash does remove excess H 2 SO 4 ; The obtained product contains 36.2% of K and 16.7% of H 2 SO 4 .
Embodiment 3
[0117] In this experiment, the potassium sulfate double salt was neutralized with ammonia according to the following reaction:
[0118] K 3 H(SO 4 ) 2 +NH4OH→K 3 (NH 4 )(SO 4 ) 2 +H 2 o
[0119] 15 g of the washed and dried double salt from Example 2 were mixed with 10 g of 25% NH 4 OH was mixed for 1 hour. The precipitate was dried at ~105°C for 2 hours. The weight of the dried crystals was 15.64 g, which contained 4.42% N and 34.2% K (compared to theoretical values of 4.3% N and 35.8% K).
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com