Preparation method of 24-epibrassinolide
A technology of epibrasinolides and compounds, which is applied in the field of preparation of plant growth regulators, can solve the problems of complicated operations, many reaction steps, and high toxicity of reagents, and achieve the effects of convenient operation, low toxicity, and low pollution
Inactive Publication Date: 2010-08-25
SHANGHAI VEGCIDES BIO FARM NANCHANG
View PDF0 Cites 18 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, when we repeated this experiment, we found that the reaction situation was not ideal, the impurity content was high, and the experimental operation was very complicated
It can be seen that the current reports on the laboratory synthesis method of 24-epibrasinolide still have many reaction steps, relatively complicated operations, and the toxicity of the reagents used can easily cause environmental pollution. It is difficult to meet the market-oriented large-scale production Require
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
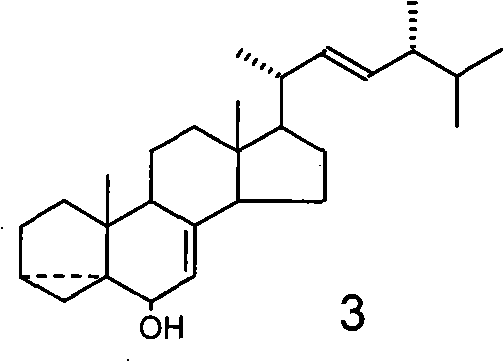
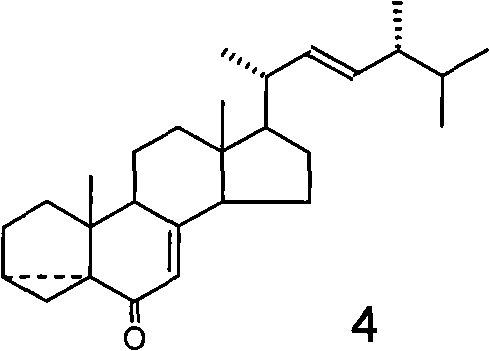
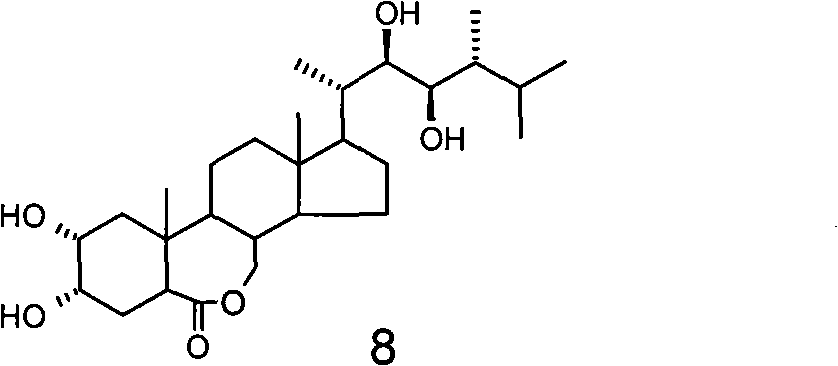
The invention relates to a preparation method of a plant growth regulator, in particular to a preparation method of 24-epibrassinolide, which belongs to the chemical field, and comprises the following steps that: 1. starting from ergosterol 1, an intermediate body 3 is prepared through two reactions, i.e. methyl sulfonyl and hydrolysis according to a traditional synthesis process; 2. the intermediate body 3 is oxidized by a novel oxidation system osmium tetroxide / inter-chloro benzoic acid peroxide to prepare an intermediate body 4; and 3. the intermediate body 4 is finally converted into the 24-epibrassinolide 8 through four reactions, i.e. reduction, rearrangement, dihydroxylation and lactonization according to the traditional synthesis process. Compared with the traditional oxidation system chromium trioxide / pyridine used in the oxidization of the intermediate body 3 into the intermediate body 4, the novel oxidation system used by the invention has the advantages of convenient operation and little pollution, and is more applicable to industrial production.
Description
Technical field The invention relates to a preparation method of a plant growth regulator, especially a preparation method of 24-epibrassinolide. It belongs to the field of chemistry. Background technique In 1979, Crove and others of the Research Institute of the United States Department of Agriculture extracted a compound with a steroidal skeleton structure from rape pollen, which was later named Brassinolide (Brassinolide, also called brassinolide) (MD Grove, GFSpencer, WK Rohwedder, N. Mandava, JF Worley, JD Warthen, Jr., GL Steffens, JLFlippen-Anderson, and JCCook, Jr., Nature (London), 1979, 281, 216). It has a very strong physiological activity, at very low concentrations (10 -9 G / L) shows a significant function of promoting plant growth and development. So far, people have isolated nearly 60 kinds of brassinolide from plants. Among them, brassinolide, 24-epibrassinolide and 28-homobrassinolide are the three most active. 24-Epibrassinolide is the first sterol compound...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07J73/00
Inventor 江民丰严兆华王锟吴中兴
Owner SHANGHAI VEGCIDES BIO FARM NANCHANG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com