Cyclic acetal base-containing thioxanthone photo initiator and preparation method thereof
A technology of thioxanthone and xanthone light, which is applied in the field of thioxanthone photoinitiator and its preparation, can solve problems such as needs, and achieve the effects of low preparation cost, reduced yellowing and toxicity, and simple synthesis
Inactive Publication Date: 2010-09-01
CHANGZHOU JIESEN CHEM MATERIAL TECH +1
View PDF0 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In order to overcome the above-mentioned defects, the technical problem to be solved in the present invention is to provide a thioxanthone photoinitiator that does not require an amine co-initiator for the existing thioxanthone photoinitiators. Initiator
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
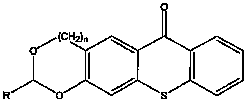
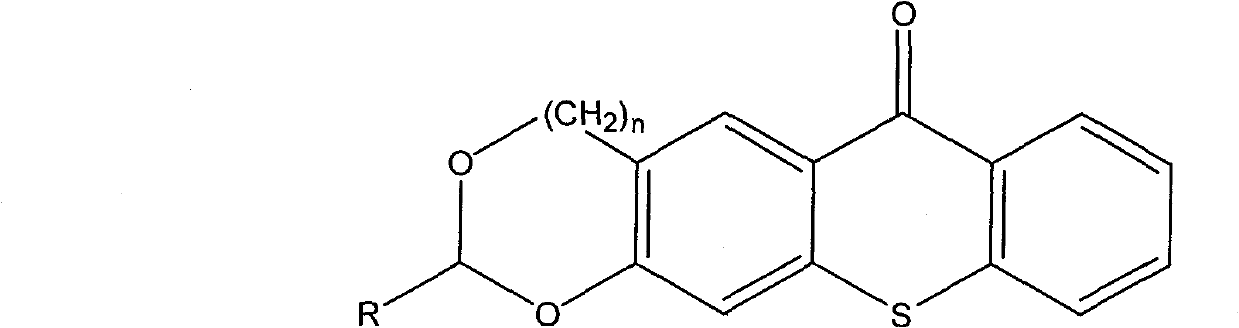
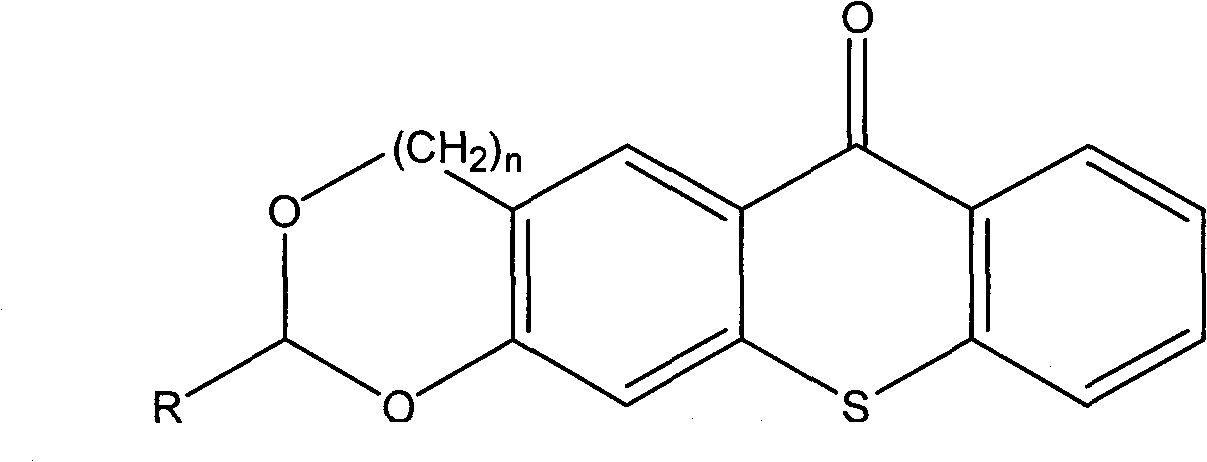
The invention relates to the technical field of photo initiators, in particular to a hioxanthone photo initiator and a preparation method thereof. The cyclic acetal base-containing thioxanthone photo initiator has a chemical structural formula shown in the specification, wherein n=0 or 1; and R=H, CH3, CH2CH3, CH2CH2CH3, CH2CH2CH2CH3, C(CH3)3, CHO, COOH, or OH, C1, F or I. The preparation method comprises the following steps of: adding one molar portion of 2-mercaptobenzoic acid into five mol portions of 1, 3-benzodioxole pentane or 1, 3-benzodioxole hexane and derivatives thereof; slowly dropwise adding concentrated acid, wherein the volume of the concentrated acid is 1.5 times the molar portion of 2-mercaptobenzoic acid calculated by L; reacting for 1 to 24 hours at the temperature of 0 to 70 DEG C; carefully pouring the mixture to boiling water , cooling and filtering; recrystallizing by utilizing a component solvent of dioxane and water; and drying in vacuum to obtain the cyclic acetal base-containing thioxanthone.
Description
technical field The invention relates to the technical field of photoinitiators, in particular to a thioxanthone photoinitiator and a preparation method thereof. Background technique The photopolymerization reaction of multifunctional monomers such as bis(meth)acrylate can quickly form a highly crosslinked polymer network structure. Especially important in areas with high sexual demands. The photoinitiator is a key component of the photocuring system, which is related to whether the oligomer and diluent of the formulation system can quickly change from liquid to solid when light is irradiated. Its basic function characteristics are: the initiator molecule has a certain light absorption ability in the ultraviolet light region (250-400nm) or visible light region (400-800nm), after directly or indirectly absorbing light energy, the initiator molecule transitions from the ground state to the excited singlet state , through intersystem crossing to the excited triplet state; af...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C08F2/48C07D495/04
Inventor 恽鹏飞赵贤顾明天顾来福王兵
Owner CHANGZHOU JIESEN CHEM MATERIAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com