Method for co-production of calcium carbonate and sodium silicate in preparation of light magnesium carbonate by chlor-alkali brine sludge
A light magnesium carbonate, calcium carbonate technology, applied in the direction of magnesium carbonate, calcium carbonate/strontium/barium, alkali metal silicate, etc. Easy operation, simple method and high economic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
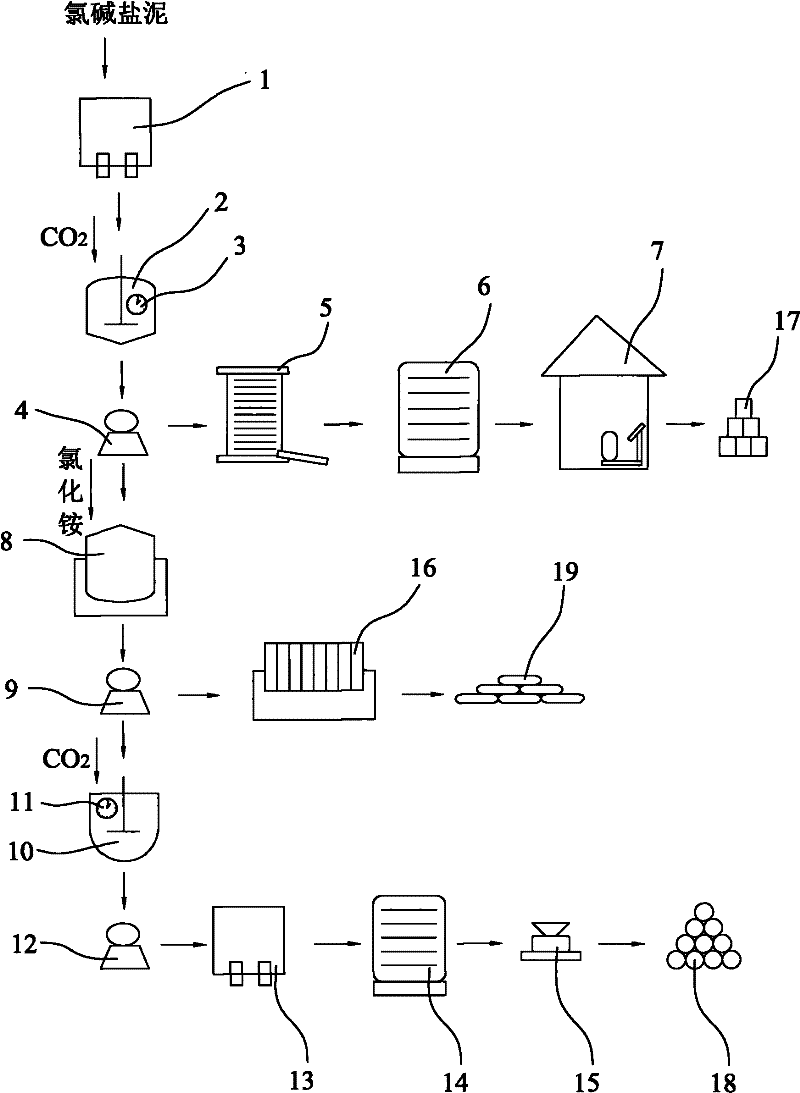
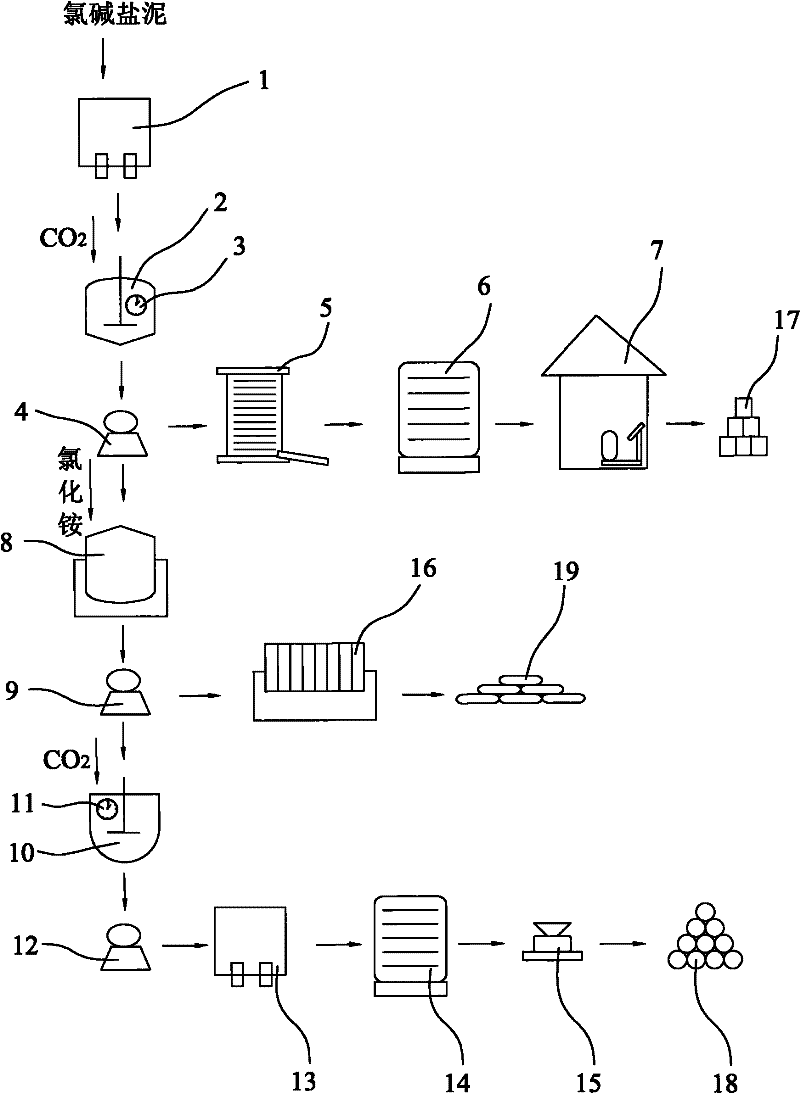
[0035] A method for producing light magnesium carbonate and co-producing calcium carbonate and water glass with chlor-alkali salt mud, comprising the steps of washing and filtering 500kg of chlor-alkali salt mud with a washing filter (1) three times to remove soluble acid radical impurities. The salt mud contains compounds mainly composed of magnesium oxide, calcium oxide and silicon dioxide; then, put the obtained salt mud into the first carbonizer (2), and pass carbon dioxide into the first carbonizer (2) Gas, so that the magnesium oxide and carbon dioxide contained in the salt mud are carbonized according to the mass ratio of pure substances 1:2.0; at this time, magnesium oxide reacts with carbon dioxide and water to produce magnesium bicarbonate or magnesium carbonate, both of which are soluble in water Calcium oxide reacts with carbon dioxide to produce calcium carbonate, which is basically insoluble in water and thus precipitates; the product obtained by the carbonization...
Embodiment 2
[0037] A method for producing light magnesium carbonate and co-producing calcium carbonate and water glass with chlor-alkali salt mud, comprising the steps of washing and filtering 500kg of chlor-alkali salt mud with a washing filter (1) three times to remove soluble acid radical impurities. The salt mud contains compounds mainly composed of magnesium oxide, calcium oxide and silicon dioxide; then, put the obtained salt mud into the first carbonizer (2), and pass carbon dioxide into the first carbonizer (2) Gas, so that the magnesium oxide and carbon dioxide contained in the salt mud are carbonized according to the mass ratio of pure substances 1:1.8; at this time, magnesium oxide reacts with carbon dioxide and water to produce magnesium bicarbonate or magnesium carbonate, both of which are soluble in water Calcium oxide reacts with carbon dioxide to produce calcium carbonate, which is basically insoluble in water and thus precipitates; the product obtained by the carbonization r...
Embodiment 3
[0039] A method for producing light magnesium carbonate and co-producing calcium carbonate and water glass with chlor-alkali salt mud, comprising the steps of washing and filtering 500kg of chlor-alkali salt mud with a washing filter (1) three times to remove soluble acid radical impurities. The salt mud contains compounds mainly composed of magnesium oxide, calcium oxide and silicon dioxide; then, put the obtained salt mud into the first carbonizer (2), and pass carbon dioxide into the first carbonizer (2) Gas, so that the magnesium oxide and carbon dioxide contained in the salt mud are carbonized according to the mass ratio of pure substances 1:2.2; at this time, magnesium oxide reacts with carbon dioxide and water to produce magnesium bicarbonate or magnesium carbonate, both of which are soluble in water Calcium oxide reacts with carbon dioxide to produce calcium carbonate, which is basically insoluble in water and thus precipitates; the product obtained by the carbonization...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com