Salen Zn (II) coordination compound and preparation method and application thereof
A complex, diaminopyridine technology, applied in the field of Salen Zn complexes and their preparation, can solve the problems of reducing the recognition effect between substrates and enzymes, and achieve the effects of shortening reaction time, reducing in vivo rejection, and optimizing reaction conditions
Inactive Publication Date: 2010-09-15
SUN YAT SEN UNIV
View PDF1 Cites 12 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
They can cause changes in the conformation of the enzyme by interacting with reverse transcriptase, thereby reducing the recognition of the substrate and the enzyme
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
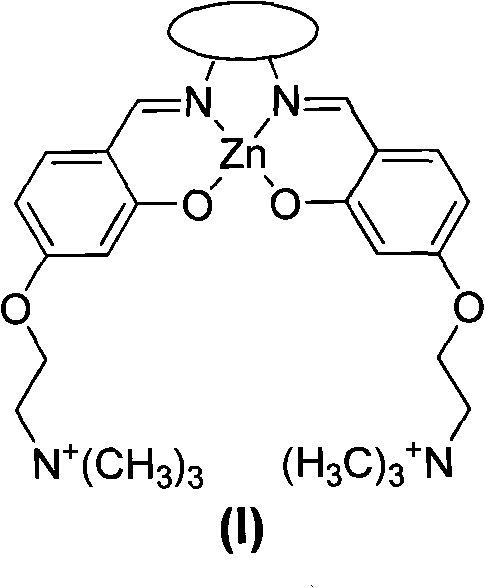
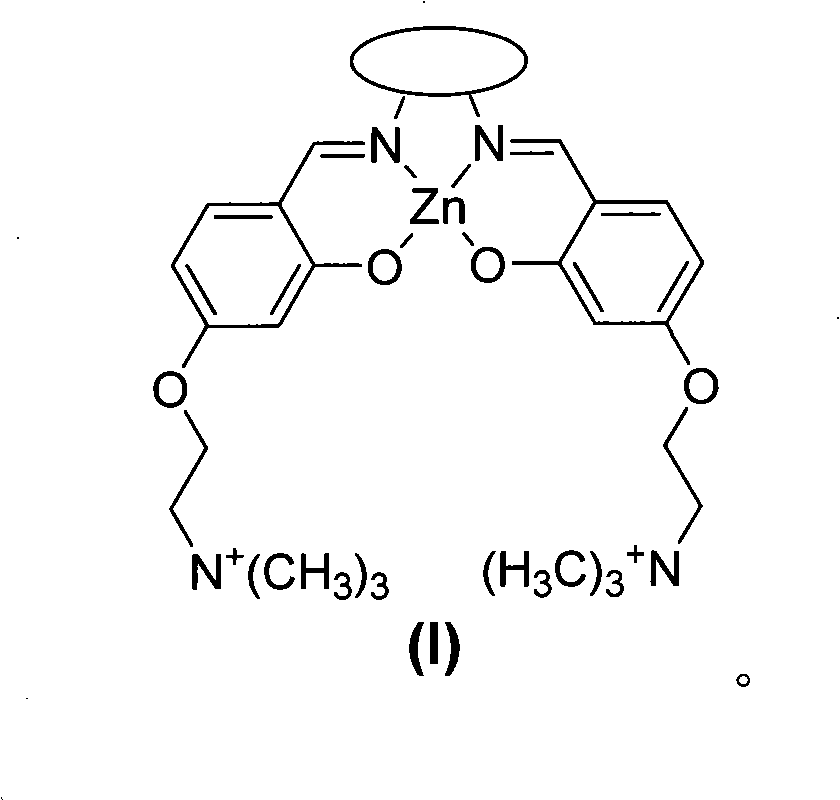
The invention discloses a Salen Zn (II) coordination compound and a preparation method and application thereof. The Salen Zn (II) coordination compound has a cation structure as shown in a general formula (I). The invention optimizes a preparation process of a Salen metal coordination compound, shortens the reaction time and enhances the productivity; and the obtained Salen Zn (II) coordination compound has very good water solubility. The Salen Zn (II) coordination compound forms a coordination bond by utilizing the axial coordination capability of the Salen metal coordination compound and an electron donor contained in a single chain part basic group of an RNA (Ribonucleic Acid) structure and is an RNA composite reagent which has more affinity compared with electrostatic interaction and weak interaction, i.e. hydrophobic interaction, hydrogen bond, Pi-Pistacking, and the like, thereby more effectively blocking the reverse transcription of a reverse transcriptase on virus RNA and providing a certain theoretical foundation for the development of drugs for treating major diseases (such as Aids, cancers, leukemia, and the like) caused by reverse transcriptase viruses.
Description
technical field The invention relates to an RNA binding reagent or an RNA reverse transcriptase inhibitor, in particular to a Salen Zn(II) complex and a preparation method and application thereof. Background technique A "retrovirus" is a virus that carries the enzyme reverse transcriptase. Unlike most living things, this virus does not have DNA, but RNA as its genetic material. When these viruses invade host cells, they can also transcribe genetic information from single-stranded viral RNA to DNA. This process is opposite to the direction of general transcription, so it is called "reverse transcription", and the enzyme that catalyzes this process is called "reverse transcriptase". Under the action of reverse transcriptase, the virus first converts RNA into cDNA, and then proliferates under the actions of DNA replication, transcription, and translation. Retroviruses are RNA viruses, which are directly responsible for the generation and spread of diseases such as AIDS and l...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07C251/24C07C249/02C07C255/61C07C253/30C07D213/74A61K31/137A61K31/277A61K31/44A61P31/18A61P35/00A61P35/02
Inventor 高峰陈星周竹新计亮年
Owner SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com