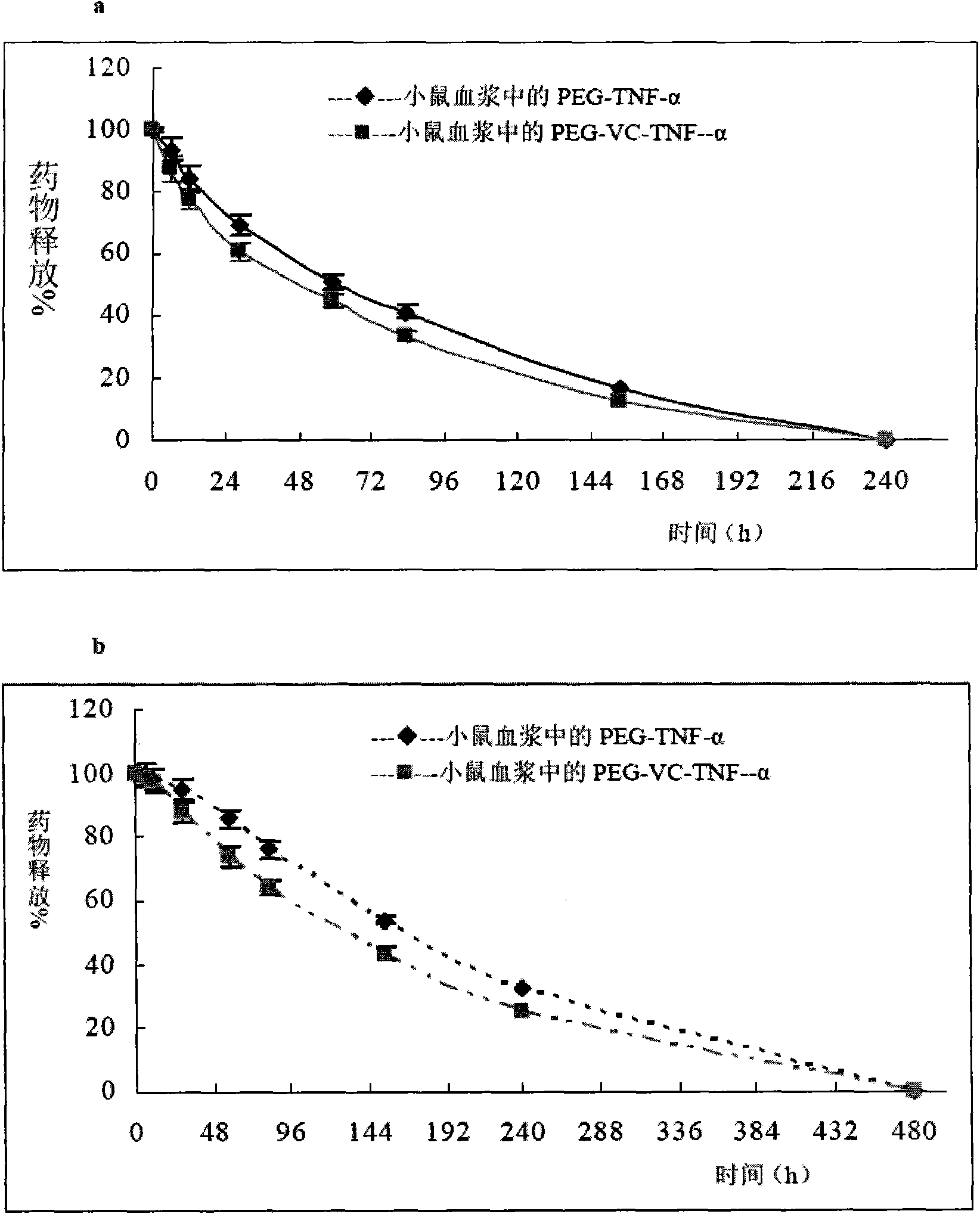
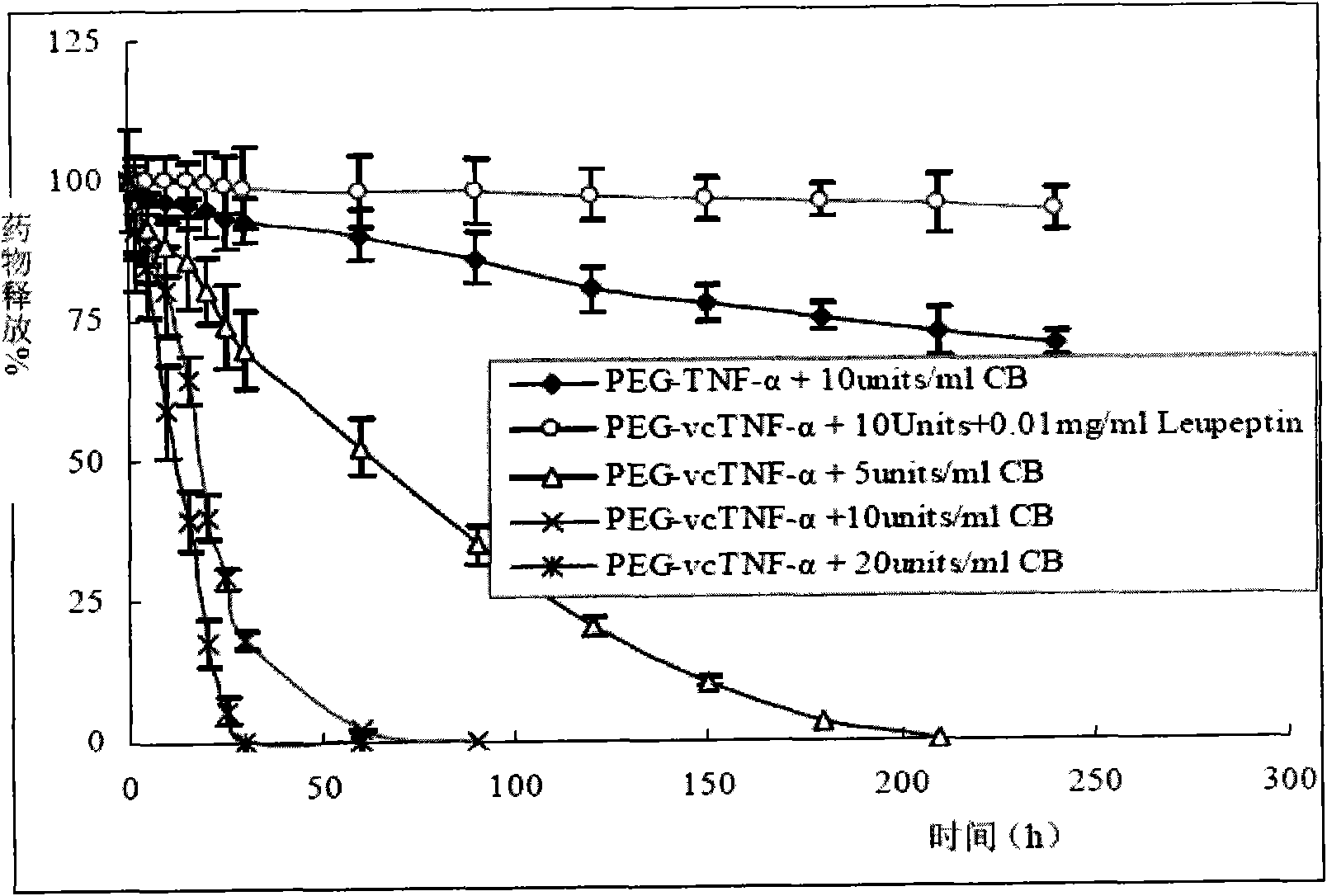
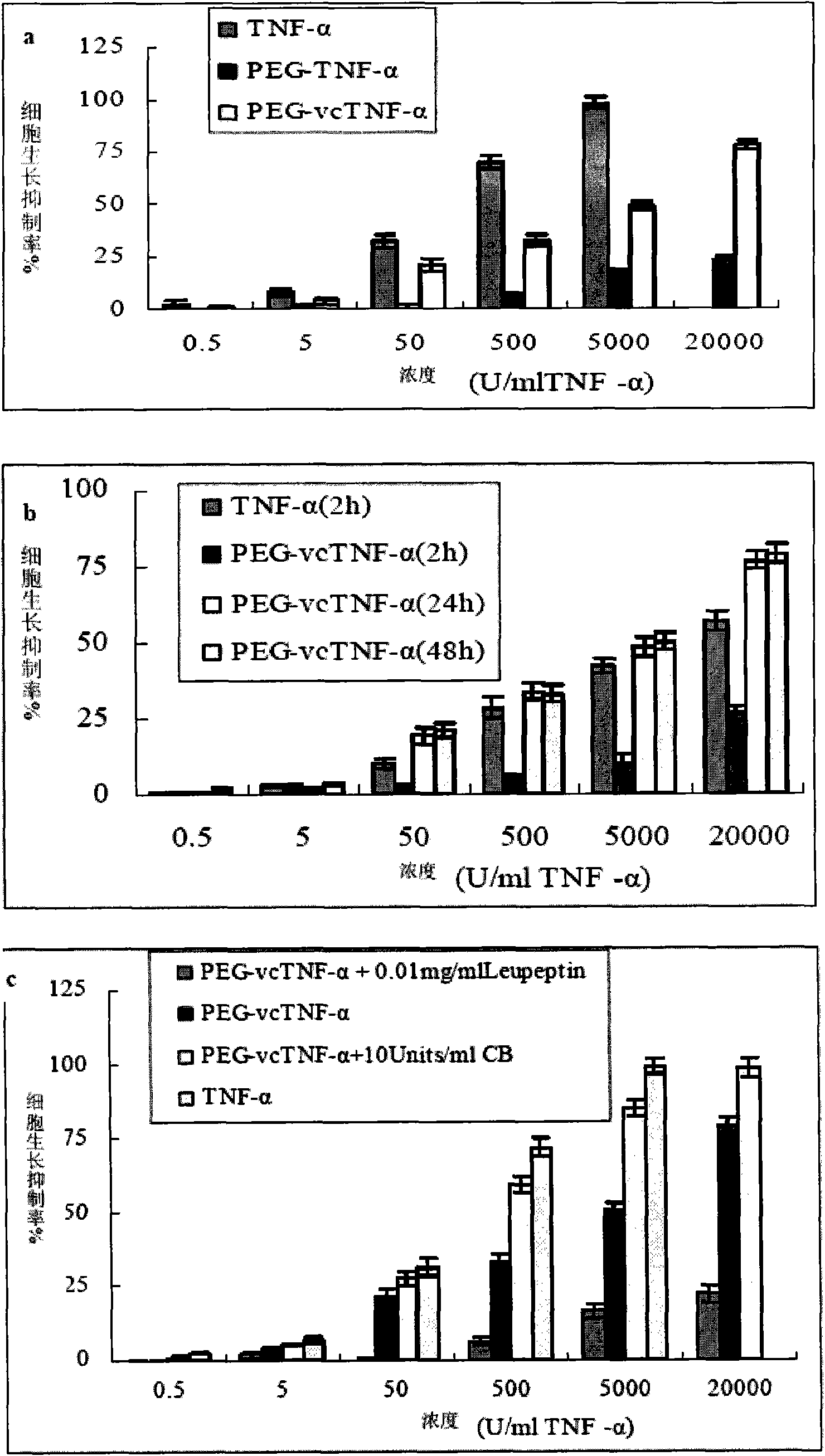
Polyethylene glycol-dipeptide-antitumour drug complex and use thereof
An anti-tumor drug, polyethylene glycol technology, used in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve problems such as limited effect and reduced half-life of conjugates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of the activated PEG-X-Y of embodiment 1, X-Y is Val-Cit
[0038] Synthesized by conventional amide condensation reaction, the easily available mPEG-NHS (the average molecular weight of PEG is 4kD) and the dipeptide (Val-Cit) according to the feeding ratio of 2:1, adding pH7.5 phosphate, HEPES or Tris-HCl and other buffer solutions were dissolved, the reaction temperature was 25° C., stirred slowly for 60 minutes, and 5 times the molar amount of PEG of glycine was added to terminate the reaction. The mPEG-Val-Cit molecular complex can be obtained after dialysis purification and lyophilization. Then use conventional EDC and NHS reagents to activate the terminal carboxyl group of the mPEG-Val-Cit molecular complex to obtain mPEG-Val-Cit-NHS.
Embodiment 2
[0039] The preparation of the activated PEG-X-Y of embodiment 2, X-Y is Ala-Lys
[0040] Synthesized by conventional amide condensation reaction, the readily available mPEG-NHS and dipeptide (Ala-Lys) are dissolved in buffers such as phosphate, HEPES or Tris-HCl at pH 7.5 at a feed ratio of 2:1 , the reaction temperature was 25° C., the mixture was stirred slowly for 60 minutes, and glycine with 5 times the molar amount of PEG was added to terminate the reaction. The mPEG-Ala-Lys molecular complex can be obtained after dialysis purification and lyophilization. Then use conventional EDC and NHS reagents to activate the terminal carboxyl group of the mPEG-XX molecular complex to obtain mPEG-Ala-Lys-NHS.
Embodiment 3
[0041] The preparation of the activated PEG-X-Y of embodiment 3, X-Y is Val-Lys
[0042] It is synthesized by conventional amide condensation reaction, and the readily available mPEG-NHS and dipeptide (Val-Lys) are dissolved in buffers such as phosphate, HEPES or Tris-HCl at pH 7.5 at a feed ratio of 1:1. , the reaction temperature was 25° C., the mixture was stirred slowly for 60 minutes, and glycine with 5 times the molar amount of PEG was added to terminate the reaction. The mPEG-Val-Lys molecular complex can be obtained after dialysis purification and lyophilization. Then, conventional EDC and NHS reagents are used to activate the terminal carboxyl group of the mPEG-Val-Lys molecular complex to obtain mPEG-Val-Lys-NHS.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com