Green synthesizing method of aryl bromide
A green synthesis technology of brominated aromatics, applied in the preparation of halogenated hydrocarbons, organic chemical methods, chemical instruments and methods, etc., can solve the problems that are not suitable for the bromination of weakly activated aromatic compounds, achieve a wide range of applications, and avoid organic solvents the effect of using
Active Publication Date: 2010-10-13
ZHEJIANG UNIV OF TECH
View PDF2 Cites 16 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Compared with the traditional method of adding bromine dropwise, these methods have fundamentally improved the atom economy, but the cost of oxidant consumption and the use of volatile solvents in the reaction are the unfavorable factors of these methods
In addition, these methods are only suitable for the bromination of activated aromatic compounds, not weakly activated aromatic compounds such as toluene
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
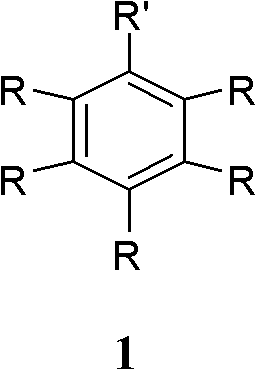
The invention discloses a green synthesizing method of aryl bromide. The synthesizing method comprises the step of carrying out bromination on an aromatic compound of which the structure is shown as a formula (1) to prepare the aryl bromide by using hydrogen bromide as a brominating agent, using copper nitrate as a catalyst and using molecular oxygen as an oxidant, wherein in the formula (I), R is hydrogen; substituent groups R1, R2, R3, R4 and R5 are respectively independently selected from hydrogen, hydroxy, amino, C1 to C8 alkoxy, single C1 to C8 alkyl amino, double C1 to C8 alkyl amino, C1 to C12 alkyl, C6 to C12 aryl, C1 to C8 acyloxy, C1 to C8 acylamino, halogen, nitryl, cyan, carboxyl and C1 to C8 acyl or C1 to C8 carbalkoxyl group; and at least one substituent group of R1, R2, R3,R4 and R5 is hydroxy, amino, C1 to C8 alkoxy, single C1 to C8 alkyl amino, double C1 to C8 alkyl amino, C1 to C8 acyloxy, C1 to C8 acylamino or C1 to C12 alkyl. The synthesizing method of the invention has the advantages of wide application range and high atom utilization, avoids using an organic solvent and has the characteristics of economy and environment protection.
Description
(1) Technical field The invention relates to a synthesis method of brominated aromatics, in particular to the catalytic bromination reaction of activated and weakly activated aromatic compounds. (2) Background technology Brominated aromatic compounds have important applications in pharmaceutical synthesis and chemical industry, such as: brominated aromatic compounds can form C-C bonds through Grignard reactions, and form aromatic-aromatic coupling compounds through coupling reactions. The preparation of brominated aromatic compounds usually requires bromine to be added to the organic solution of aromatic compounds, and the reaction generates hydrogen bromide in an equimolar amount with the bromine participating in the reaction. The bromine atom utilization rate of this method is only 50%. Caused a waste of bromine. At the same time, the organic solvents used in large quantities in the reaction pollute the environment. Therefore, it is desirable to improve the atomic utili...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07B39/00C07C39/27C07C37/62C07C41/22C07C43/225C07C17/156C07C25/02C07C205/26C07C201/12C07C211/52C07C209/74C07C255/53C07C253/30C07C25/125C07C69/63C07C43/29
Inventor 李景华王剑刘锐
Owner ZHEJIANG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com