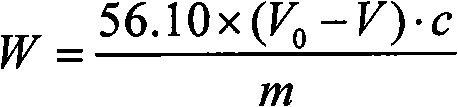Method for measuring content of phenolic hydroxyl groups in polyphenyl ether by titrimetry
A polyphenylene ether and phenolic hydroxyl technology, which is applied in the detection field of titration to determine the content of phenolic hydroxyl in polyphenylene ether, can solve the problems of difficult titration and cumbersome operation of the titration process, and achieve simple operation, simple experimental equipment, and measurement high precision effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] For a polyphenylene ether sample with a number average molecular weight of about 12400, the steps for measuring its phenolic hydroxyl content by titration are as follows:
[0043] 1) Accurately weigh 5.0000 g of a polyphenylene ether sample with an electronic analytical balance, put it in a 250 mL Erlenmeyer flask, add 50 mL of chloroform, and stir to dissolve.
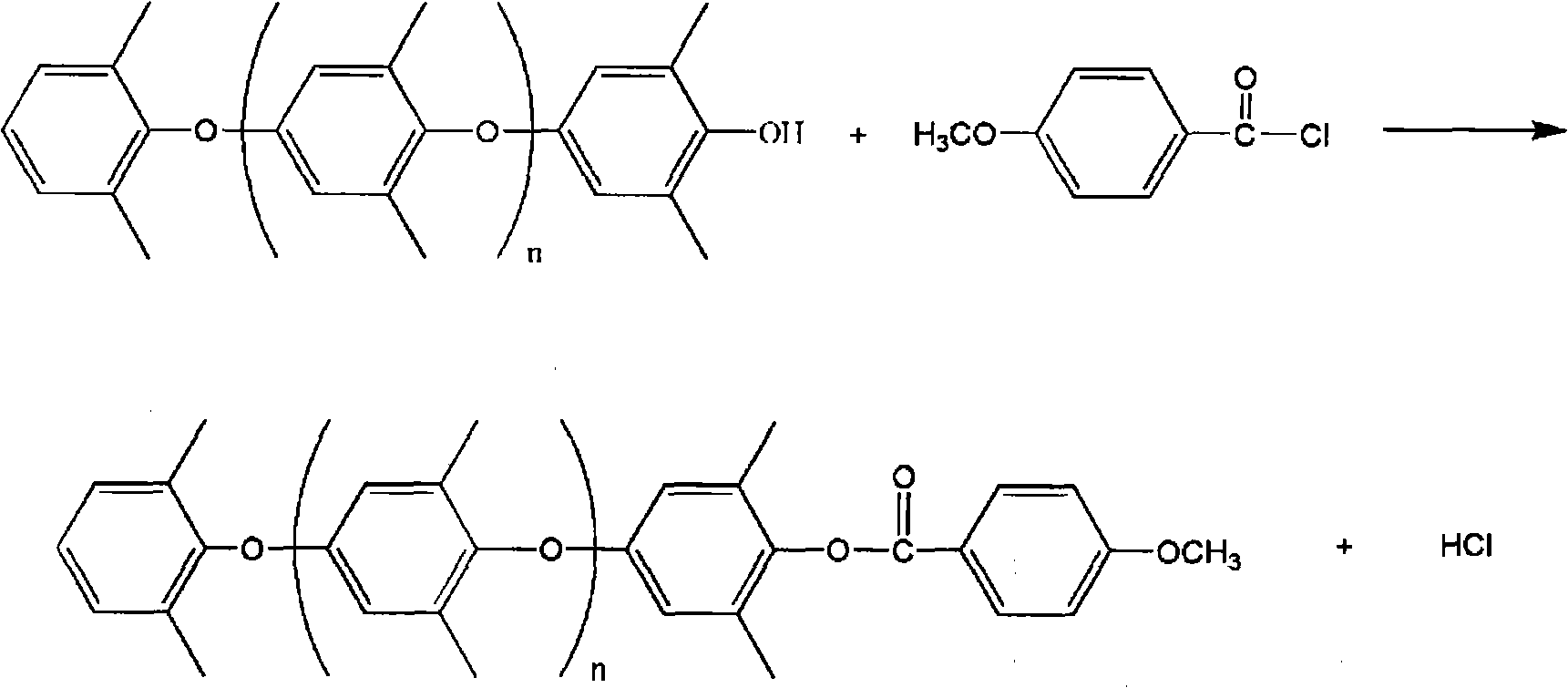
[0044] 2) Add 0.05 g of 4-methoxybenzoyl chloride, heat to reflux for 30 minutes (temperature 55° C.), and then cool to room temperature.
[0045] 3) Add 10ml of deionized water to the Erlenmeyer flask, stir for 10min, add 10mL of deionized water from the top of the condenser tube to rinse the inner wall of the condenser tube, and then remove the condenser tube.
[0046] 4) Add 4 drops of phenolphthalein indicator solution to the Erlenmeyer flask, and titrate with standard KOH aqueous solution under stirring until the system turns pink and remains unchanged for 30 seconds, which is the end point.
[0047] 5) R...
Embodiment 2
[0051] For a polyphenylene ether sample with a number average molecular weight of about 5200, the steps for measuring its phenolic hydroxyl content by titration are as follows:
[0052] 1) Accurately weigh 5.0000 g of a polyphenylene ether sample with an electronic analytical balance, put it in a 250 mL Erlenmeyer flask, add 50 mL of chloroform, and stir to dissolve.
[0053] 2) Add 0.05 g of 4-methoxybenzoyl chloride, heat to reflux for 30 minutes (temperature 50° C.), and then cool to room temperature.
[0054] 3) Add 10ml of deionized water to the Erlenmeyer flask, stir for 10min, add 10mL of deionized water from the top of the condenser tube to rinse the inner wall of the condenser tube, and then remove the condenser tube.
[0055] 4) Add 4 drops of phenolphthalein indicator solution to the Erlenmeyer flask, and titrate with standard KOH aqueous solution under stirring until the system turns pink and remains unchanged for 30 seconds, which is the end point.
[0056] 5) Re...
Embodiment 3
[0060] For a polyphenylene ether sample with a number average molecular weight of about 1200, the steps for measuring its phenolic hydroxyl content by titration are as follows:
[0061] 1) Accurately weigh 5.0000 g of a polyphenylene ether sample with an electronic analytical balance, put it in a 250 mL Erlenmeyer flask, add 50 mL of chloroform, and stir to dissolve.
[0062] 2) Add 0.05 g of 4-methoxybenzoyl chloride, heat to reflux for 30 minutes (temperature 60° C.), and then cool to room temperature.
[0063] 3) Add 10ml of deionized water to the Erlenmeyer flask, stir for 10min, add 10mL of deionized water from the top of the condenser tube to rinse the inner wall of the condenser tube, and then remove the condenser tube.
[0064] 4) Add 4 drops of phenolphthalein indicator solution to the Erlenmeyer flask, and titrate with standard KOH aqueous solution under stirring until the system turns pink and remains unchanged for 30 seconds, which is the end point.
[0065] 5) Re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com