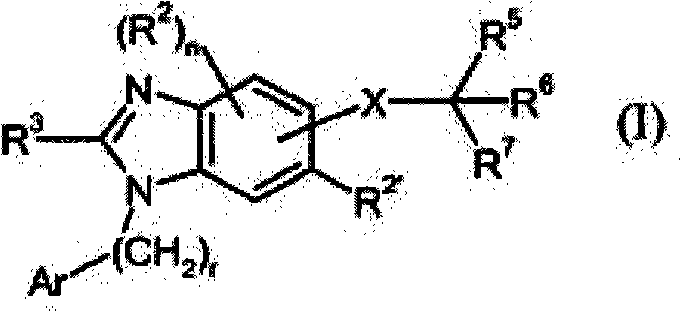
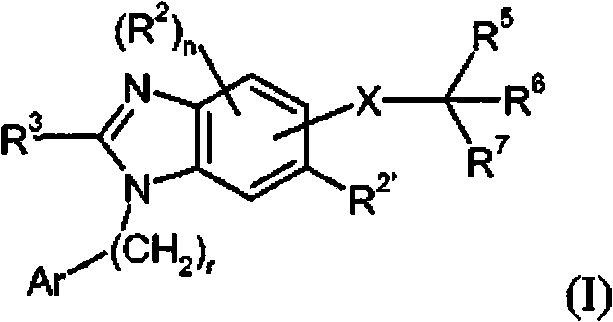
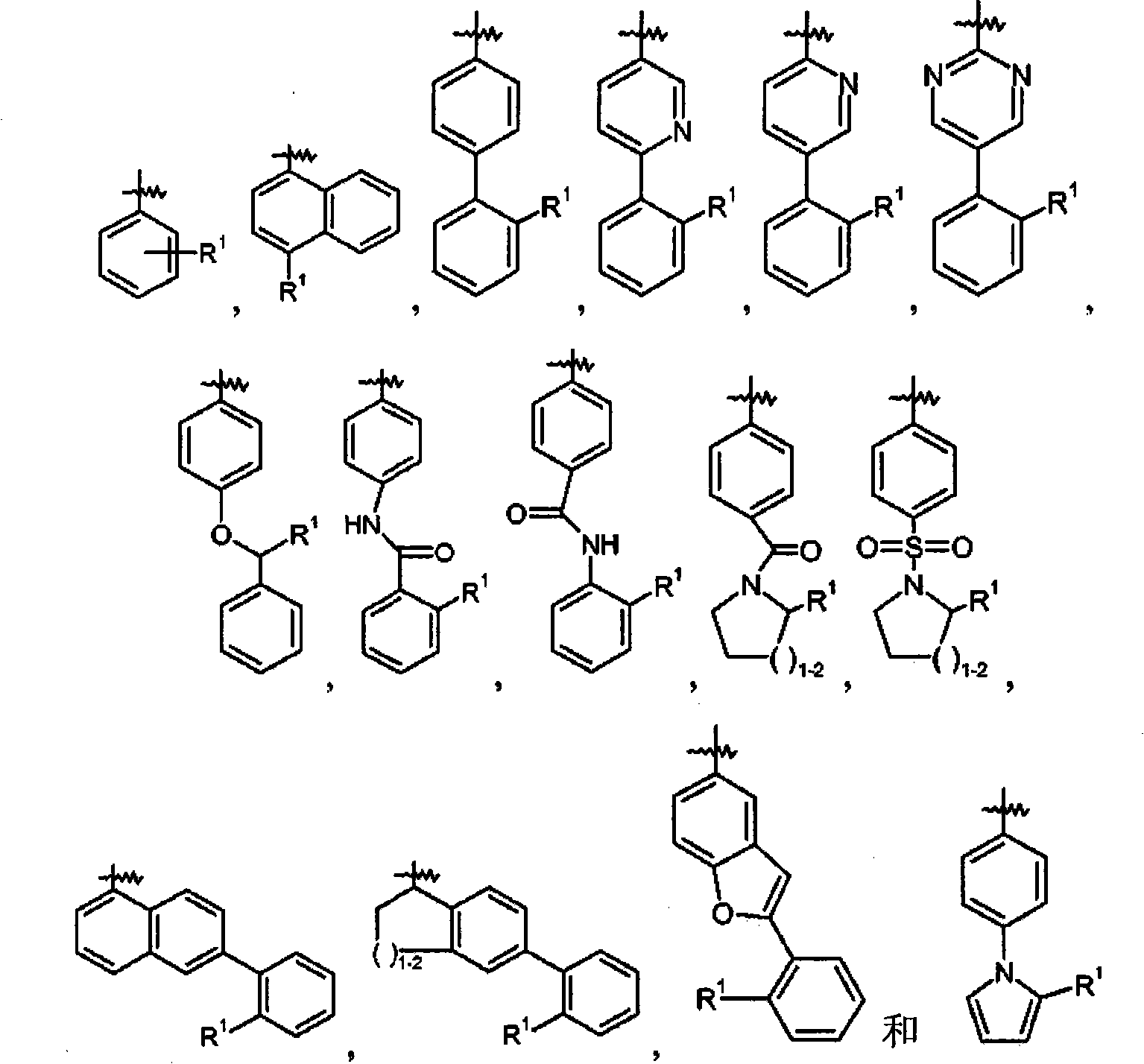
Dual-acting benzoimidazole derivative and their use as antihypertensive agents
A compound, alkyl technology, applied in the field of disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0327] Preparation of compound (2)
[0328] Compound (2) can be readily synthesized by the following techniques described in the literature, for example, Neustadt et al. (1994) J. Med. Chem. 37: 2461-2476 and Moree et al. (1995) J. Org. Chem. 60:5157-69, and synthesized by using the exemplary procedures set forth below. Examples of depicted achiral compounds (2) include:
[0329]
[0330] Since compound (2) has a chiral center, it is desirable to synthesize specific stereoisomers and examples are provided below.
[0331] Chiral amino hydroxamate compound (2 i ) preparation
[0332]
[0333] To a solution of compound (2a) in DMF containing HOBt and hydroxylamine hydrochloride is added a base such as DIPEA and a coupling agent such as EDC. The mixture was stirred at room temperature until the reaction was complete, then concentrated in vacuo. The resulting material was partitioned between 5% THF in EtOAc and 1M phosphoric acid. The organic layer is collected and wash...
example
[0452] The following Preparations and Examples are provided to illustrate specific embodiments of the invention. However, unless expressly stated otherwise, these specific examples are not intended to limit the scope of the invention in any way.
[0453] Unless otherwise stated, the following abbreviations have the following meanings, and any other abbreviations used herein and not defined have their standard meanings:
[0454] ACE angiotensin converting enzyme
[0455] APP aminopeptidase P
[0456] AT 1 Angiotensin II type 1 (receptor)
[0457] AT 2 Angiotensin II type 2 (receptor)
[0458] BCA bisquinolinecarboxylic acid
[0459] BSA bovine serum albumin
[0460] DCM dichloromethane
[0461] DMF N,N-Dimethylformamide
[0462] DMSO Dimethyl Sulfoxide
[0463] Dnp 2,4-Dinitrophenyl
[0464] DOCA deoxycorticosterone acetate
[0465] EDC N-(3-Dimethylaminopropyl)-N’-ethylcarbodiimide
[0466] EDTA ethylenediaminetetraacetic acid
[0467] EGT...
example 1
[0498] 4-[6-((R)-1-Benzyl-2-hydroxycarbamoylethylcarbamoyl)-4-methyl-2-propylbenzene imidazol-1-ylmethyl]benzoylase (1-a) and 4-[5-((R)-benzyl-2-hydroxycarbamoyl ethyl Carbamoyl)-7-methyl-2-propylbenzimidazol-1-ylmethyl]benzoic acid (1-b)
[0499]
[0500] To 4-[6-((R)-1-benzyl-2-benzyloxycarbamoylethylcarbamoyl)-4-methyl-2-propylbenzimidazol-1-ylmethyl ]benzoic acid and 4-[5-((R)-1-benzyl-2-benzyloxycarbamoylethylcarbamoyl)-7-methyl-2-propylbenzimidazole-1- 10% Pd / C (200 mg) was added to a nitrogen-saturated solution of methyl]benzoic acid. The mixture was degassed and stirred overnight at room temperature under hydrogen (1 atm). mixture through diatomaceous earth It was filtered, concentrated to dryness, and purified by preparative reverse phase HPLC. The desired product, compound 1-b (TFA salt; 30 mg) was obtained as a colorless solid.
[0501] Compound 1-a: C30 h 32 N 4 o 5 ESMS[M+H] + Calculated value, 529.25; Experimental value, 529.2. Hold time (analy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com