Preparation method of swine fever virus nucleic acid standard substance
A swine fever virus and standard substance technology, which is applied in the field of preparation of swine fever virus nucleic acid standard substances, can solve the problems of restricting the accuracy of detection results, pollution, RNase pollution, unreported, etc., and achieves increased reliability and safety. High performance and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1. Select the 5' non-coding region (5'UTR) of the conserved region of the classical swine fever virus gene as the amplification target region.
[0057] 2. Design and synthesis of primers: A1: 5'-AGT ACA GGG TAG TCG TCA GTG GTT CG-3'; A2: 5'-CTG CTG TAC ATG GCA CAT GGA GTT G-3'; provided by Shanghai Yingjun Biotechnology Co., Ltd. Synthesized by the company, made up to 0.25μM with DEPC water, and stored at -20°C for future use.
[0058] 3. Amplification of the target sequence: Extract the total RNA of the virus liquid (tissue) with the TRIzol Reagent kit (purchased from Invitrogen); take 10 μl of RNA, add 2 μl of Oligo (dT) and 8 μl of reverse transcription solution (5×Buffer 4 μl, 2 μl DTT, 1 μl dNTP, 0.5 μl reverse transcriptase, 0.5 μl RNasin), reverse transcription at 42°C for 60 min and 70°C / 15 min to obtain total cDNA; take 10 μl cDNA, add 29 μl H 2 O, pre-denaturation at 95°C for 5min, add 11µl PCR solution (10×Buffer 5µl, A1 1µl, A2 1µl, dNTP 2µl, Taq enzyme 2µl...
Embodiment 2
[0063] RNA copy number determination
[0064] The prepared RNA sample was diluted 10 times and 100 times, and the absorbance value measurement results were: 10 times dilution, OD260=0.44406, the concentration was 35.5248 μg / ml; 100 times dilution, OD260=0.03481, the concentration was 3.4827 μg / ml; Calculated from the nucleic acid sequence, the molecular weight of the single-stranded template is MW=669.40kDa; after calculation, the copy number of RNA molecules per tube is: 3.11×10 12 Copies / 20 μl.
Embodiment 3
[0066] Inspection and determination of standard substance of classical swine fever virus nucleic acid
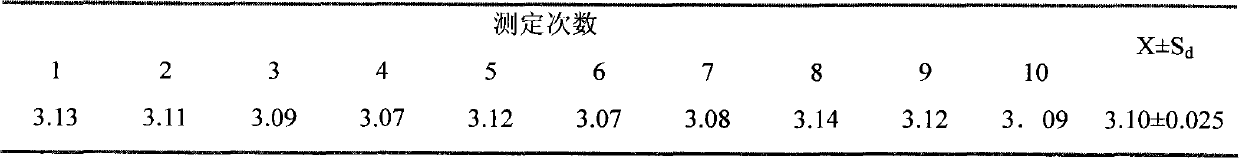
[0067] 1. Uniformity test: 10 samples were randomly selected from the samples, and the RNA copy number per unit volume of each sample was detected. The results are shown in Table 1. After statistical analysis, there was no significant difference among the samples, indicating that the batch of samples had good uniformity.
[0068] Table 1 RNA sample copy number (×10 12 copy / 20μl)
[0069]
[0070] 2. Stability test: Samples were randomly selected every other week for testing, and the test results after 11 weeks were not significantly different from the test results in the first month, indicating that the sample has good stability. It is speculated that its stable storage period is 5 years above.
[0071] 3. Collaborative calibration: Distribute the samples to relevant domestic institutions, and let each unit detect the RNA copy number per unit volume of the sample recei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com