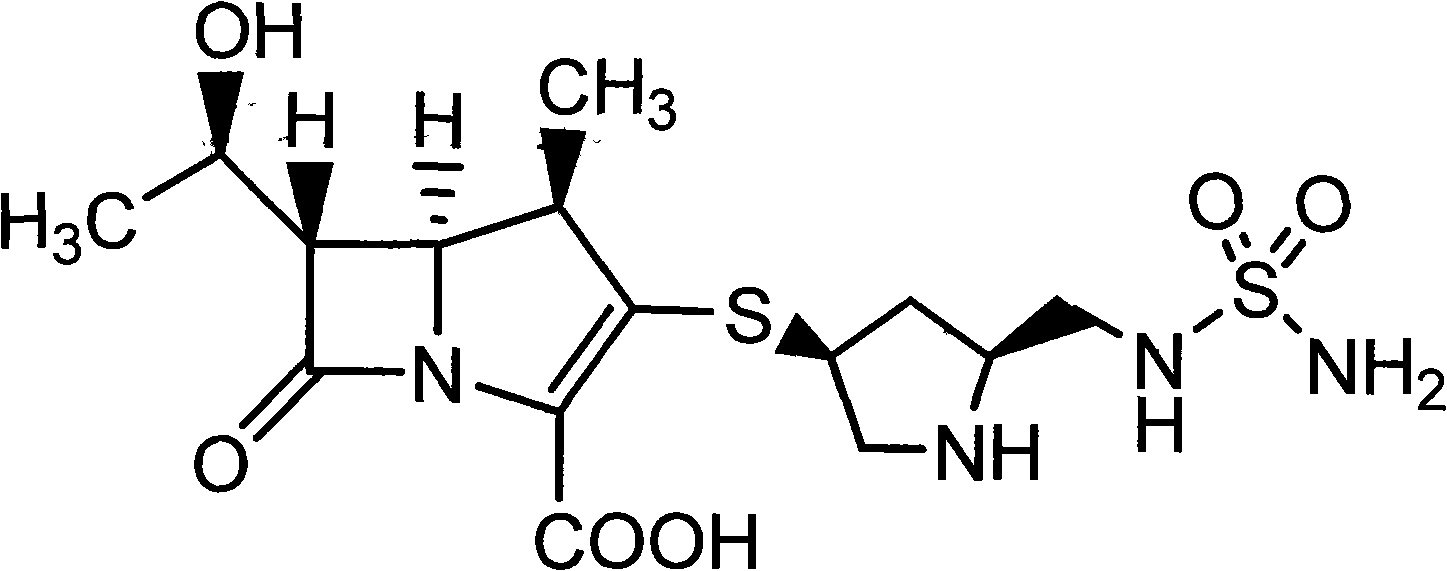
Composition of Doripenem and amino acid
A technology of composition and amino acid, which is applied in the direction of drug delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve problems such as darkening of color, poor fluidity of crystalline powder, and difficulty in ensuring the level of sterility , to achieve the effect of improving stability and storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 (preparation of doripenem crude product)
[0042]
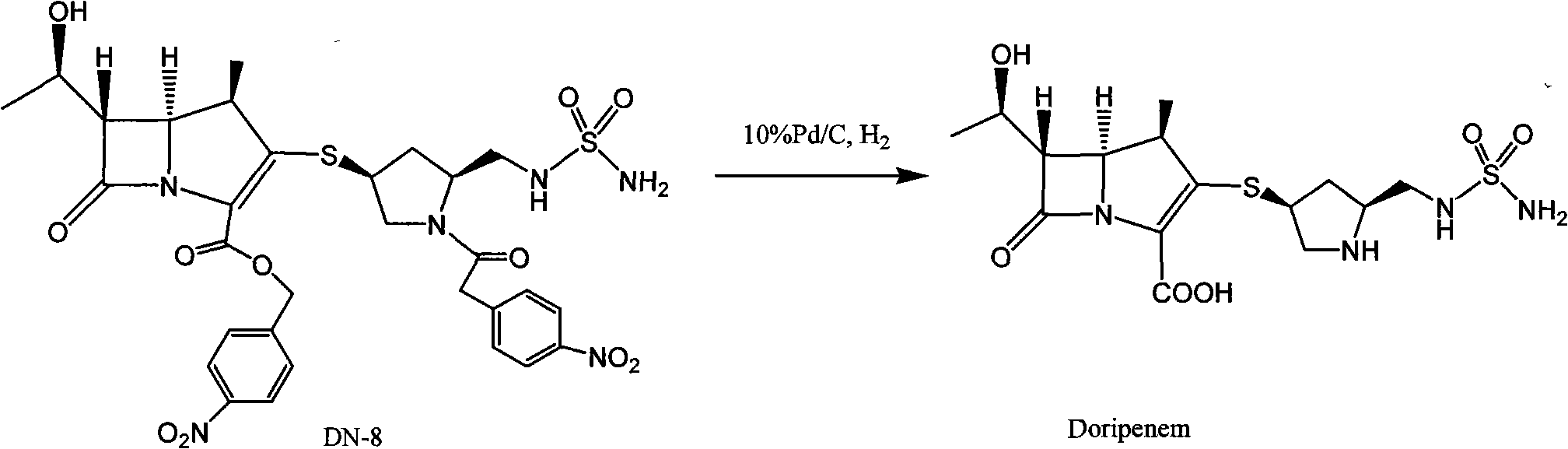
[0043] 100 grams of DN-8 (referring to document organic process research & development 2003, 7, 649-654 method preparation) is dissolved in water (450ml) and THF (800ml), adds 100 grams of Pd / C (water content 66%), heats to At 25°C, under the pressure of 20-24kg, react for 2 hours, heat is released during hydrogenation, and the reaction temperature is maintained at 30-40°C. After the reaction is completed, filter with suction and wash with 150ml (THF:water=2:1) Palladium carbon, wash the water layer with ethyl acetate (750ml×2), wash the water layer with n-butanol (500ml×2), cool the water layer to 0°C, a light yellow solid precipitates, keep warm at 0-5°C and stir overnight, pump Filter and dry under reduced pressure at 25°C for 15 hours to obtain crude doripenem (32 g, yield 65%, purity 96.2%).
Embodiment 2
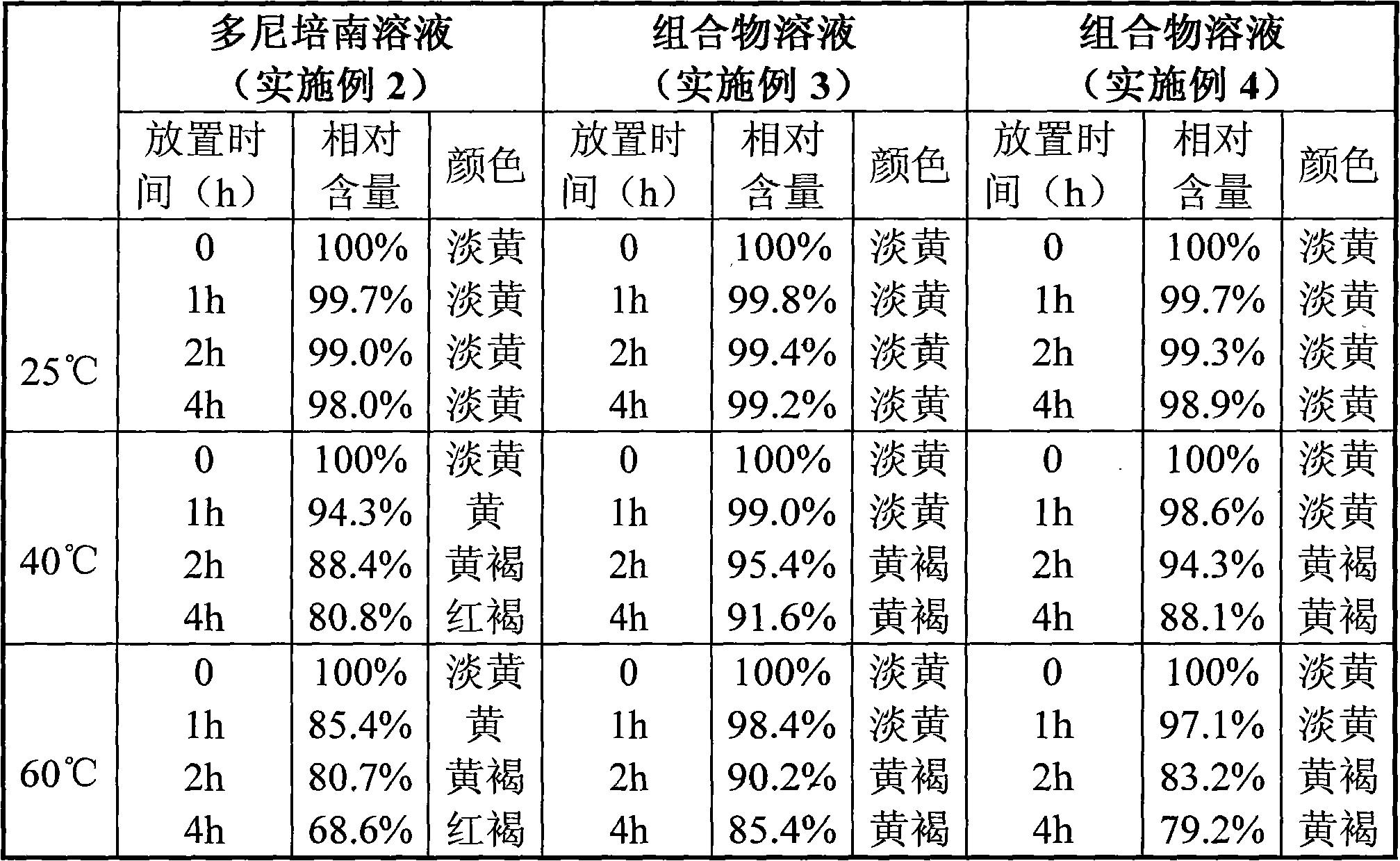
[0045] Dissolve the crude product of doripenem (1.0g) in distilled aqueous solution (22ml), heat with stirring, and dissolve at 55°C, keep the solution at 50-55°C, add activated carbon (50mg) and stir for decolorization for 5min, filter, Remove the heat source, take the filtrate and cool it to 0-5°C and stir it. After about 10 hours, solids will precipitate out, and keep it at 0-5°C for aging for 2 hours. Depyrogenated ethanol (30ml) was dropped into it within about 1 hour, kept at 0-5°C for aging for 2 hours, and then the obtained crystals were filtered out. The obtained crystals were washed with 80% aqueous ethanol (5 ml), and then dried under reduced pressure at 30°C (20-30 mmHg) for 4 hours to obtain 0.62 g of doripenem (purity: 98.51%).
Embodiment 3
[0047] Crude doripenem (1.0g) and L-threonine (0.9g) were dissolved in distilled aqueous solution (15ml), heated with stirring, and dissolved at 50°C, the solution was maintained at 50°C, and activated carbon ( 50 mg) was stirred for 5 minutes to decolorize, filtered, and the heat source was removed, and the filtrate was cooled to 0-5°C and stirred. Solids precipitated within about 1 hour, and aged at 0-5°C for 2 hours. Depyrogenated ethanol (30ml) was dropped into it within about 1 hour, kept at 0-5°C for aging for 2 hours, and then the obtained crystals were filtered out. The obtained crystals were washed with 80% ethanol aqueous solution (5ml), and dried under reduced pressure at 30°C (20-30mmHg) for 4 hours to obtain 1.7g of the composition of doripenem and L-threonine (donipenem The purity is 99.68%, and the mass ratio of doripenem to L-threonine is 1:1).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com