Adrenocorticotropic hormone analogs and related methods
A technology of analogs and adrenal membranes, applied in the direction of adrenocorticotropic hormone, chemical instruments and methods, drug combinations, etc., can solve problems such as impaired immune function, excessive production of adrenal stress hormones, and reduced growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
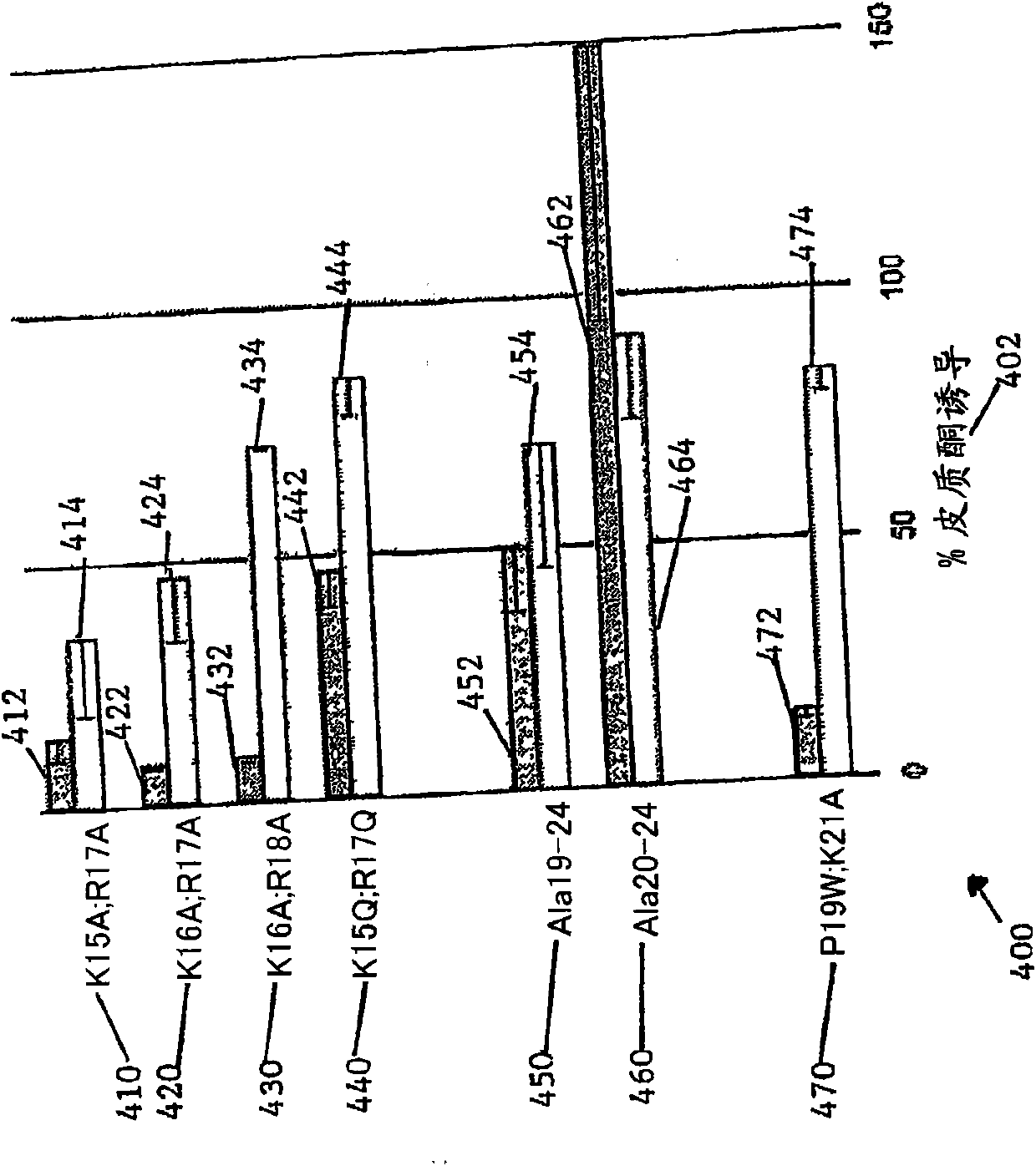
[0193] Example 1 Serum corticosteroid induction assay (in vivo)
[0194] ACTH analogs having reduced ACTH-mediated corticosteroid secretion are identified by performing an in vivo serum corticosteroid induction assay to measure corticosteroid levels in the subject's blood following administration of the ACTH analog.
[0195] In vivo serum corticosteroid induction assays were performed according to the following method. First, dexamethasone (0.4mg / 0.1ml PBS per mouse; Sigma, St.Louis, MO ) to inhibit their endogenous ACTH production. See Hajos, GT et al., "Studies of the potency of polypeptides with ACTH action by a new method based on continuous measurement of plasma corticosterone," Steroids Lipids Res 3:225-228 (1972); Costa, JL et al., "Mutational Analysis of evolutionarily conserved ACTH residues, "Gen Comp Endocrinol, 136:12-16 (2004); and Karpac, J et al., "Development, Maintenance, and Function of the Adrenal Gland in Early Postnatal Pro-opiomelanocortin Null Mutant M...
Embodiment 2
[0198] Example 2 Serum corticosteroid inhibition assay (in vivo)
[0199] ACTH analogs with reduced ACTH-mediated corticosteroid secretion were identified by testing their ability to inhibit adrenal hormone production induced by unmodified ACTH by performing an in vivo serum corticosteroid inhibition assay. Corticosteroid levels in the blood of a subject are measured following administration of an ACTH analog test compound in combination with a compound having known corticosteroid-inducing activity (eg, ACTH or another ACTH analog). The ACTH analog test compound can be administered before, simultaneously with, or after the administration of the corticosteroid-producing compound.
[0200] In vivo serum corticosteroid suppression assays were performed as follows. ACTH analog test compounds, such as those in Table 1 with low activity, were tested for their ability to inhibit adrenal hormone production induced by unmodified ACTH.
[0201] First, dexamethasone (0.4mg / 0.1ml PBS pe...
Embodiment 3
[0205] Example 3 Adrenal binding assay (in vitro)
[0206] ACTH analogs with the ability to bind adrenal receptors can be identified by performing an in vitro adrenal binding assay. The amount of radiolabeled ACTH analog test compound bound to explanted adrenal membranes can be determined to identify ACTH analogs with adrenal receptor binding activity. Preferably, this is performed in serum-free medium (in vitro serum-free adrenal binding assay).
[0207] In vitro adrenal competitive binding assays can also identify ACTH analogs that have the ability to bind adrenal receptors with a higher binding affinity than compounds known to have adrenal binding activity (preferably ACTH), In the in vitro adrenal gland competitive binding assay, the reduction in the amount of radiolabeled compound that binds to the adrenal gland (such as ACTH) is measured by measuring the non-radiolabeled ACTH analog test compound in the medium containing explanted adrenal gland membranes. Different con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com