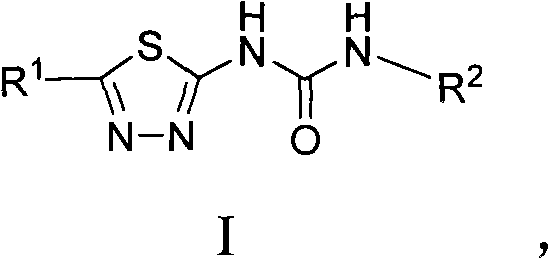
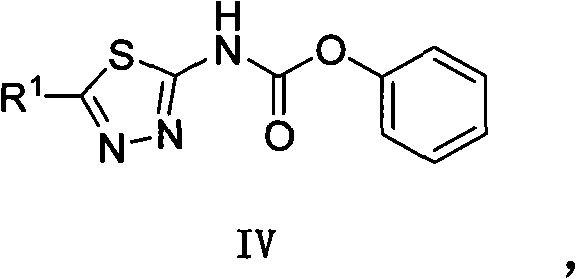
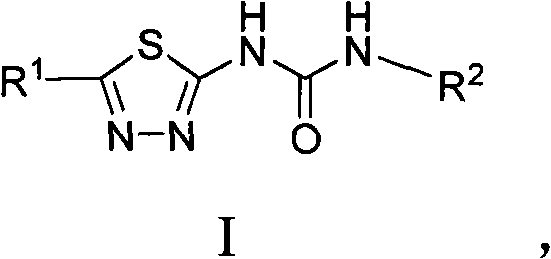
Asymmetric heterocyclic ring substituent urea compound and preparation method and application thereof
A substituted urea, asymmetric technology, applied in the field of asymmetric heterocyclic substituted urea compounds, can solve problems such as unsafe production, and achieve the effect of a safe and non-toxic preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] This example illustrates the preparation of (5-trifluoromethyl-1,3,4-thiadiazol-2-yl)phenylcarbamate
[0079]
[0080] 6g (0.355mol) NaH was added into a 250mL round-bottomed flask containing 25mL THF, and 5-trifluoromethyl-1,3,4-thiadiazol-2-amine (20g , 0.118mol) into a 100mL constant-pressure dropping funnel, and added dropwise under an ice bath. After the dropwise addition was completed, the reaction was continued for 15 min under ice bath, and then diphenyl carbonate (25.33 g, 0.118 mol) was added, and the reaction was continued for 5 hours under ice bath, overnight at 20°C. After the reaction was completed, 100 mL of ethyl acetate was added to the reaction solution, the organic phase was separated, and then washed 3 times with saturated brine (50 mL each time), the organic phase was dried with anhydrous sodium sulfate, spin-dried the solvent, and washed with petroleum Ether / ethyl acetate=1:1 (volume ratio) was separated through a silica gel column to obtain (5...
Embodiment 2
[0082] This example illustrates the preparation of 1-(pyridin-2-yl)-3-(5-trifluoromethyl-1,3,4-thiadiazol-2-yl)urea (compound 1)
[0083] (5-trifluoromethyl-1,3,4-thiadiazol-2-yl)carbamate phenyl ester (500mg, 1.73mmol) and 2-pyridinamine (195.3mg , 2.075mmol) and 2mL THF were added into a microwave reaction bottle, and the microwave reaction bottle was placed in an Initiator Biotage microwave reactor with a reaction temperature of 150°C and a reaction time of 25 minutes. After the reaction, a large amount of white solid precipitated, filtered, washed with tetrahydrofuran, and dried at room temperature to obtain compound 1, a total of 280.2 mg, yield 56.4%, white solid, m.p.225-227 ° C, 1 H NMR [DMSO, 400MHz]: δ=12.859(s, 1H, NH), 10.123(s, 1H, NH), 8.35(d, J=4Hz, 1H), 7.861-7.817(m, 1H), 7.528( d, J=8.4Hz, 1H), 7.149-7.118 (t, J=6.2Hz, 1H); ESI-MS: (M - )m / z(%)=287(100); theoretical value: C 9 h 6 f 3 N 5 OS (289.2): C, 37.37; H, 2.09; N, 24.21, actual value: C, 37.27; ...
Embodiment 3
[0085] This example illustrates the preparation of 1-(6-methylpyridin-2-yl)-3-(5-trifluoromethyl-1,3,4-thiadiazol-2-yl)urea (compound 2)
[0086] (5-trifluoromethyl-1,3,4-thiadiazol-2-yl)carbamate phenyl ester (500mg, 1.73mmol) and 6-picoline-2 prepared according to the method of Example 1 - Amine (187mg, 1.73mmol) and 2mL THF were added to a microwave reaction vial, and the microwave reaction vial was placed in an Initiator Biotage microwave reactor with a reaction temperature of 150°C and a reaction time of 25 minutes. After the reaction, a large amount of white solid precipitated, filtered, washed with tetrahydrofuran, and dried at room temperature to obtain compound 2, 240.2mg, yield 55.0%, white solid, m.p.238-240°C, 1 H NMR [DMSO, 300MHz] δ=13.056(s, 1H, NH), 10.130(s, 1H, NH), 7.746(t, J=10.6Hz, 1H), 7.330(d, J=11.2Hz, 1H) , 7.016 (d, J=10.0Hz, 1H), 2.476 (s, 3H, CH3); ESI-MS: (M - )m / z(%)=302(100); theoretical value: C 10 h 8 f 3 N 5 OS.(303.3): C, 39.6; H, 2.66;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com