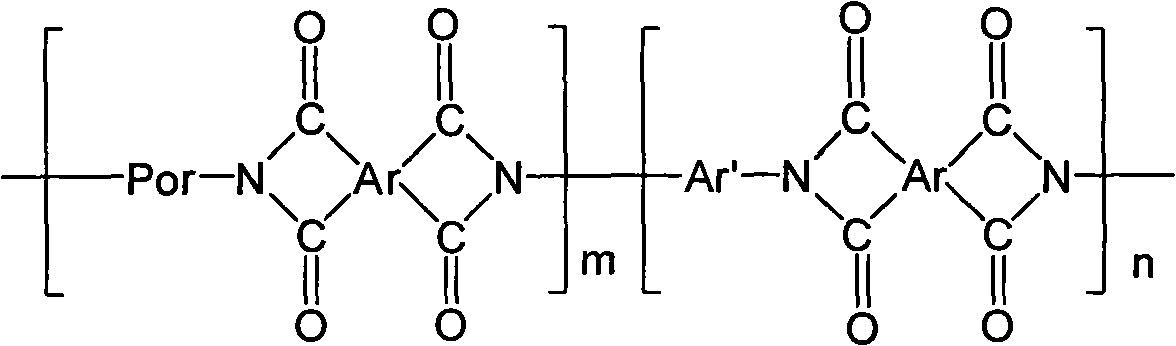
Polyimide containing porphyrin as well as preparation method and application thereof
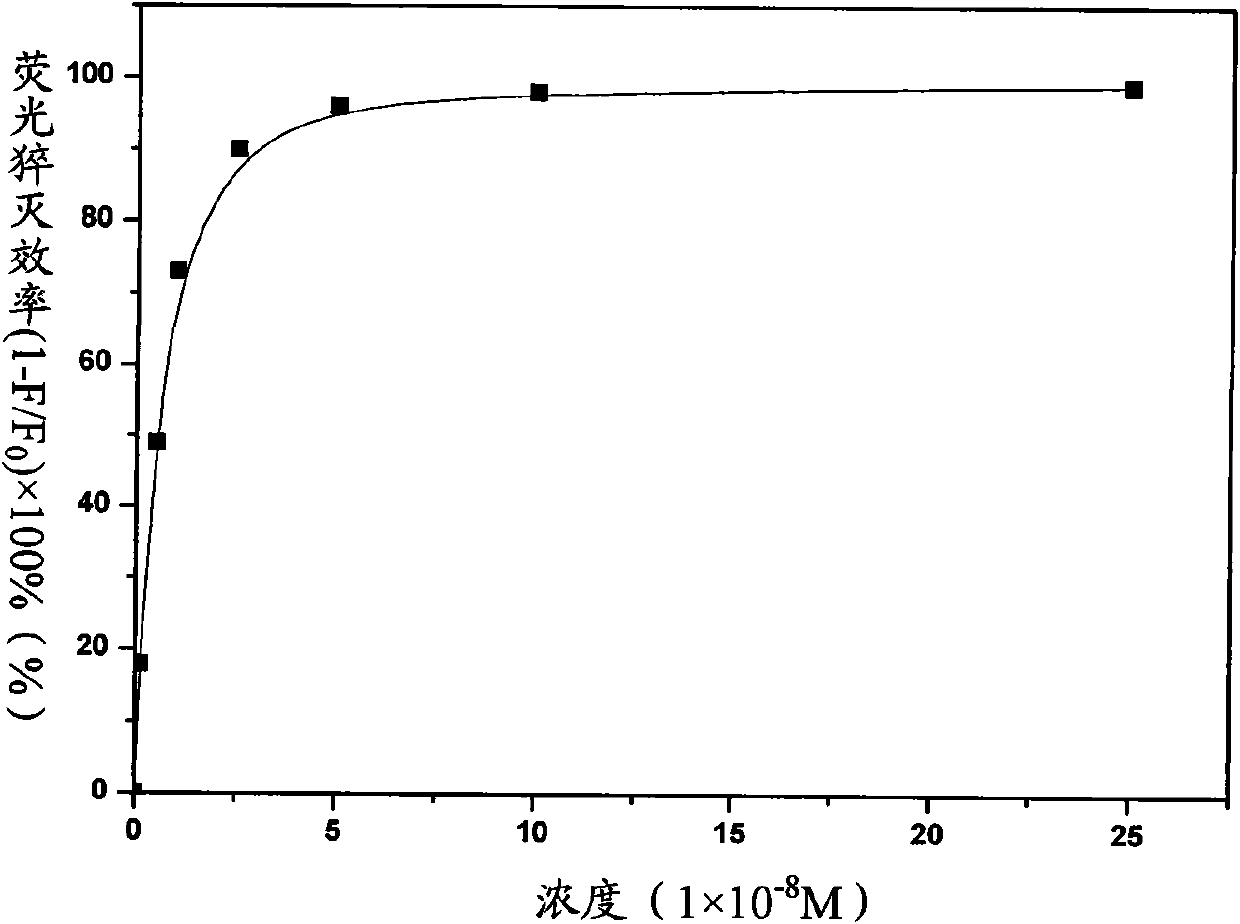
A technology of polyimide and polyamic acid, which is applied in the field of porphyrin functionalized polymer compounds, can solve problems such as the application of outdated materials and the ineffective use of porphyrin photoelectric properties, and achieve easy sampling, processing performance and chemical stability Good performance, avoid the effect of fluorescence quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] (1) Synthesis of porphyrin-containing polyimide
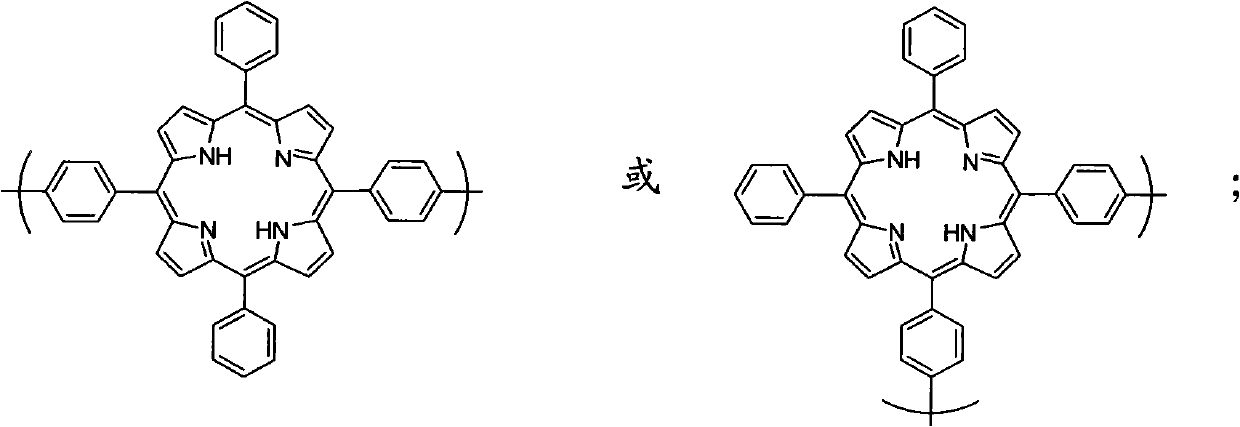
[0061] 5,15-bis(4-aminophenyl)-10,20-diphenylporphyrin (trans-DATPP) or 5,10-bis(4-aminophenyl)-15,20-diphenylporphyrin Preparation of (cis-DATPP) monomer: 10 mL of trifluoroacetic acid (TFA) and 2.65 mmol of sodium nitrite were added to 0.326 mmol of tetraphenylporphyrin (TPP), and 100 mL of water was added after magnetic stirring at room temperature for 90 seconds. Chloromethane extraction (6 times), 25 mL each time, the extracted dichloromethane organic layer was washed with saturated aqueous sodium bicarbonate solution (100 mL), dried over anhydrous sodium sulfate for 24 h, and evaporated to dryness to obtain a solid. Then 3.55 mmol of stannous chloride and 50 mL of hydrochloric acid were added, heated to 65° C. for 1 h under magnetic stirring, and then the reaction was stopped. After the system is cooled, add 100 mL of water, neutralize the solution with ammonia water to pH = 8, extract with dichloromethane (6 time...
Embodiment 2
[0070] (1) Synthesis of porphyrin-containing polyimide
[0071] The preparation of 5,10-bis(4-aminophenyl)-15,20-diphenylporphyrin (cis-DATPP) is the same as in Example 1.
[0072] Under nitrogen protection, 5,10-bis(4-aminophenyl)-15,20-diphenylporphyrin (cis-DATPP) and 4,4′-diaminodiphenyl ether (ODA) were used as The diamine monomer is dissolved in N,N-dimethylacetamide (DMAc), the molar ratio of cis-DATPP and ODA is 0.1:1, and it is dissolved by electromagnetic stirring to obtain a homogeneous system, and then mixed with the two diamines 4,4′-hexafluoroisopropylidene-phthalic anhydride (6FDA) monomers in equimolar amounts of the total molar amount of the amine monomers are added to the reaction system twice, and the interval between the two additions is half an hour; The ratio of the added amount of 4,4'-hexafluoroisopropylidene-phthalic anhydride (6FDA) to N,N-dimethylacetamide (DMAc) was 50 mg / mL. After continuing to react in an ice-water bath for 5 h, the reaction was...
Embodiment 3
[0080] The preparation of 5,15-bis(4-aminophenyl)-10,20-diphenylporphyrin (trans-DATPP) is the same as in Example 1.
[0081] Under nitrogen protection, 5,15-bis(4-aminophenyl)-10,20-diphenylporphyrin (trans-DATPP) and 4,4′-(hexafluoroisopropylidene) bis Aniline (6FDAM) was dissolved in N,N-dimethylacetamide (DMAc) as a diamine monomer, and the molar ratio of trans-DATPP to ODA was 0.25:1. Electromagnetic stirring was used to dissolve it to obtain a homogeneous system, and then 4,4'-(2,2,2-trifluoro-1-phenylethylene) diphthalic anhydride (3FDA) monomer in an equimolar amount to the total molar amount of the two diamine monomers was added to the In the reaction system, the interval between two additions is half an hour; 4,4'-(2,2,2-trifluoro-1-phenylethylene) diphthalic anhydride (3FDA) Amide (DMAc) was added at a ratio of 50 mg / mL. After continuing to react in an ice-water bath for 6 h, the reaction was continued for 22 h at room temperature to generate porphyrin-containing ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com