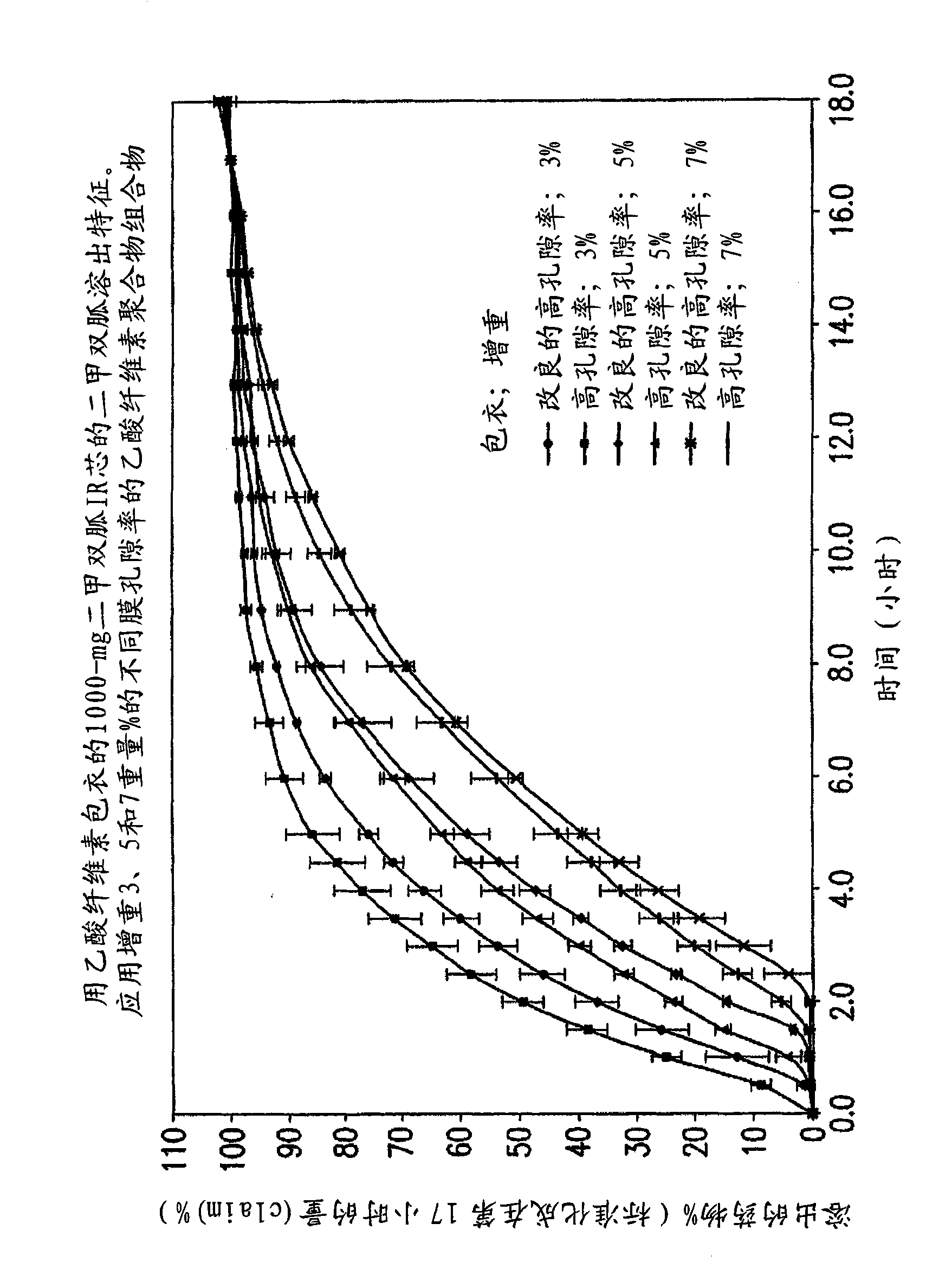
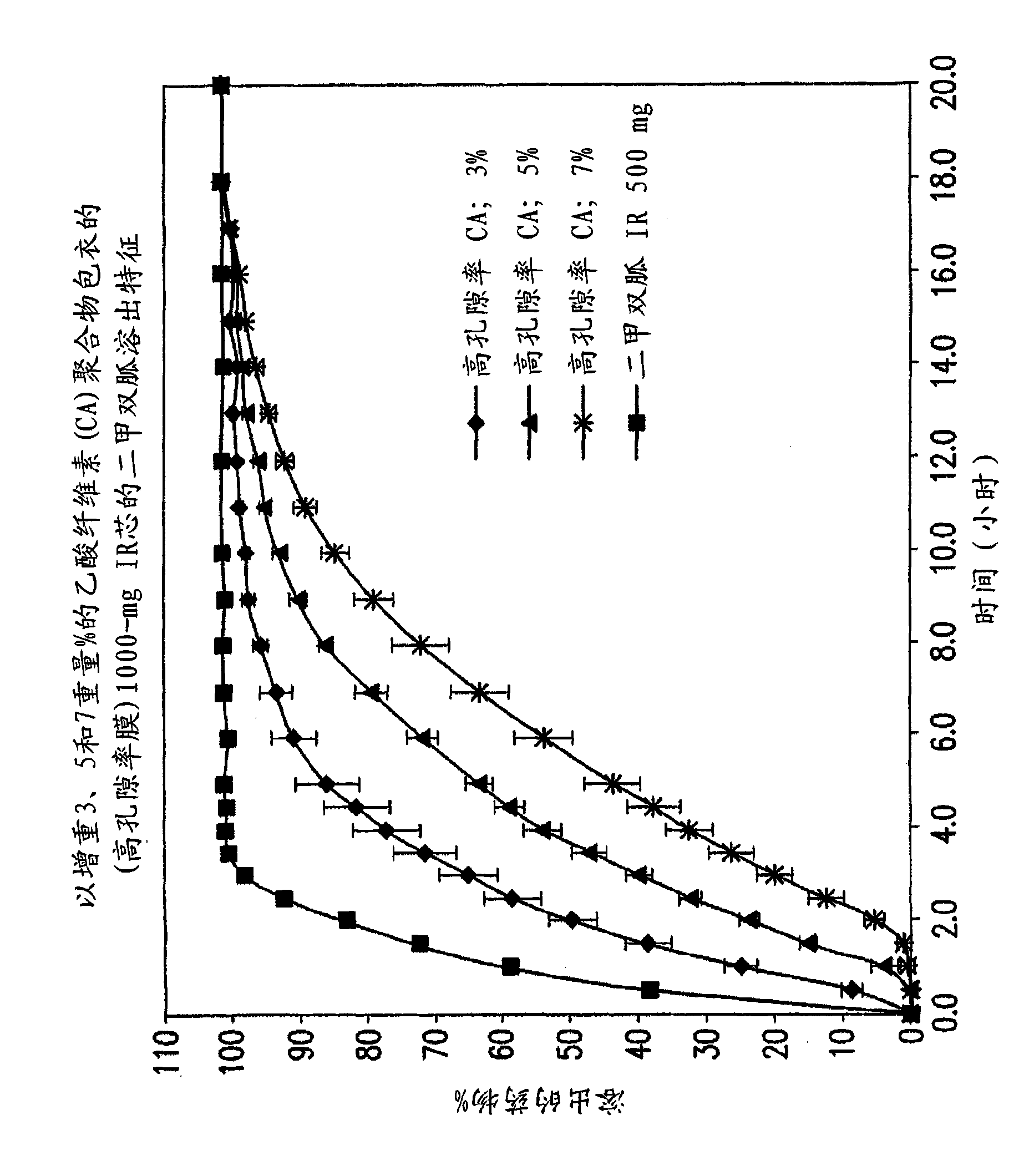
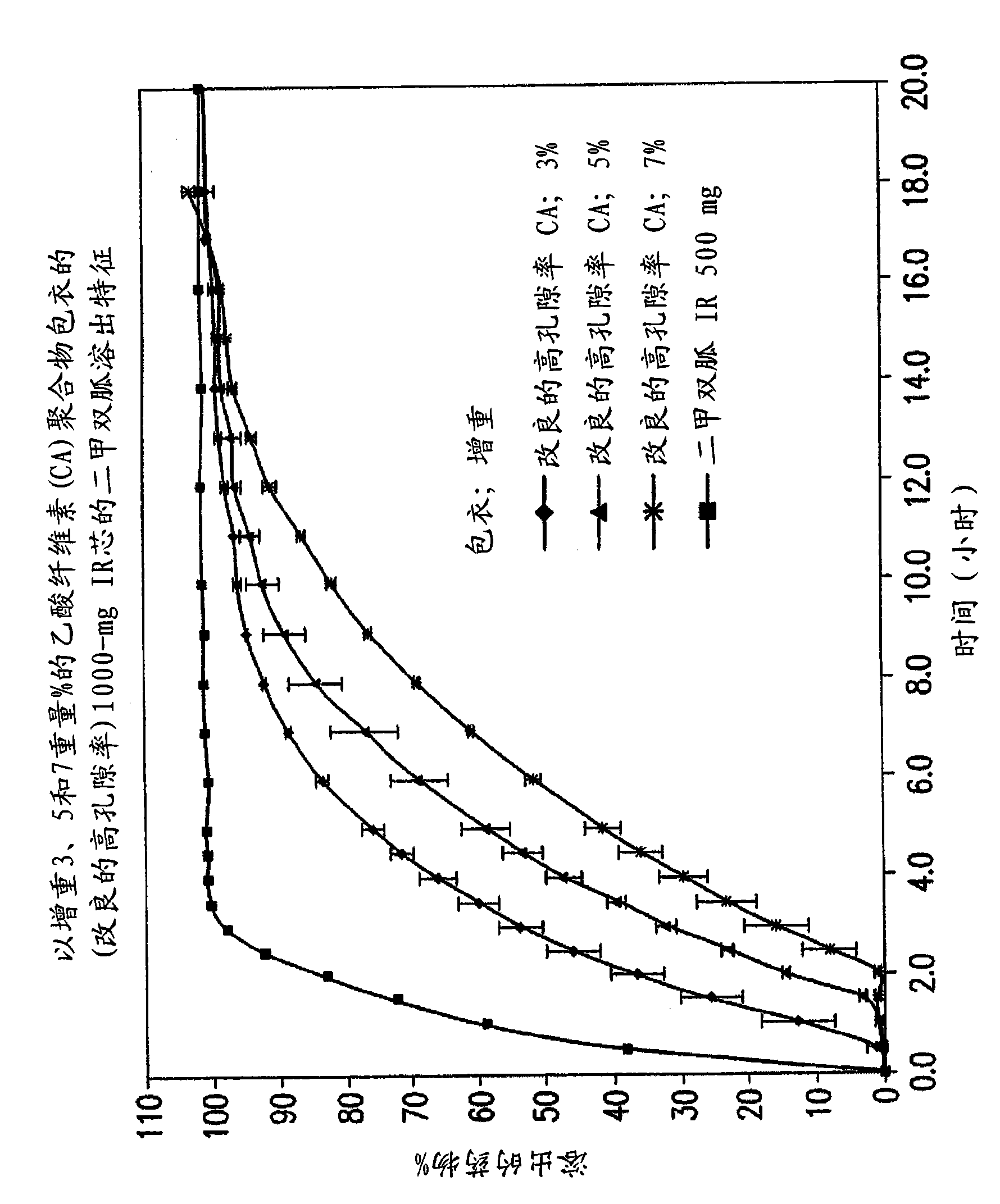
Pharmaceutical compositions of a combination of metformin and a dipeptidyl peptidase-IV inhibitor
A technology of metformin hydrochloride and composition, which is applied in the field of combined pharmaceutical composition of metformin and dipeptidyl peptidase-IV inhibitor, which can solve problems such as difficult treatment plan, insufficient control of blood sugar, and complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The preparation of sitagliptin and its pharmaceutically acceptable salts is disclosed in US Patent No. 6,699,871, the entire contents of which are incorporated herein by reference. The preparation of sitagliptin phosphate monohydrate is disclosed in US Patent No. 7,326,708, the entire contents of which are incorporated herein by reference.
[0021] The unit dose concentrations of sitagliptin free base anhydrate (active moiety) for inclusion into the fixed dose combination pharmaceutical compositions of the present invention are 25, 50 and 100 mg. Sitagliptin phosphate monohydrate was used in the pharmaceutical composition in an amount equivalent to that of sitagliptin free base anhydrate, ie 32.125, 64.25 and 128.5 mg, respectively.
[0022] The unit dose concentrations of metformin hydrochloride incorporated into the fixed dose combinations of the present invention are 250, 500, 750, 850 and 1000 mg. These unit dosage concentrations of metformin hydrochloride represen...
Embodiment 1
[0112] 50 or 100 mg of sitagliptin coated with sustained release polymer (3% w / w level) and Fixed-dose combination of 1000 mg of metformin hydrochloride
[0113] Element 100 / 1000 100 / 1000 50 / 1000 50 / 1000
[0114] mg / tablet %w / w mg / tablet %w / w
[0115] 1. Chip tablet
[0116] Metformin HCl 1000 76.725 10007 6.725
[0117] PVP K29 / 32 75.27 5.775 75.27 5.775
[0118] Avicel PH 102 TM 195.50 15 195.50 15
[0119] Silica 6.517 0.5 6.517 0.5
[0120] Sodium stearyl fumarate 26.067 2.0 26.067 2.0
[0121] Total amount of chips 1303.36 100 1303.36 100
[0122] 2. Cellulose acetate
[0123] (CA) polymer bag
[0124] Clothes
[0125] CA-398-10 19.55 1.5 19.55 1.5
[0126] PEG 3350 9.775 0.75 9.775 0.75
[0127] Triacetin 9.775 0.75 9.775 0.75
[0128] Total CA SR coating 39.1 3 39.1 3
[0129] SR coated tablet 1342.46 103 1342.46 103
[0130] 3. Sitagliptin pack
[0131] Clothes
[0132] Sitagliptin phosphate 12...
Embodiment 2
[0167] 50 or 100 mg of sitagliptin and 1000 mg coated with sustained release polymer (5% w / w) fixed-dose combination of metformin hydrochloride in milligrams
[0168] Element 100 / 1000 100 / 1000 50 / 1000 50 / 1000
[0169] mg / tablet %w / w mg / tablet %w / w
[0170] 1. Chip tablet
[0171] Metformin HCl 1000 76.725 1000 76.725
[0172] PVP K29 / 32 75.27 5.775 75.27 5.775
[0173] Avicel PH 102 TM 195.50 15 195.50 15
[0174] Silica 6.517 0.5 6.517 0.5
[0175] Sodium stearyl fumarate 26.067 2.0 26.067 2.0
[0176] Total amount of chips 1303.36 100 1303.36 100
[0177] 2. Cellulose acetate
[0178] (CA)
[0179] polymer coating
[0180] CA-398-10 32.58 2.53 2.58 2.5
[0181] PEG 3350 16.29 1.25 16.29 1.25
[0182] Triacetin 16.29 1.25 16.29 1.25
[0183] Total CA SR coating 65.16 5 65.16 5
[0184] SR coated tablet 1368.42 105 1368.42 105
[0185] 3. Sitagliptin pack
[0186] Clothes
[0187] Sitagliptin phosphate 128.52* ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com