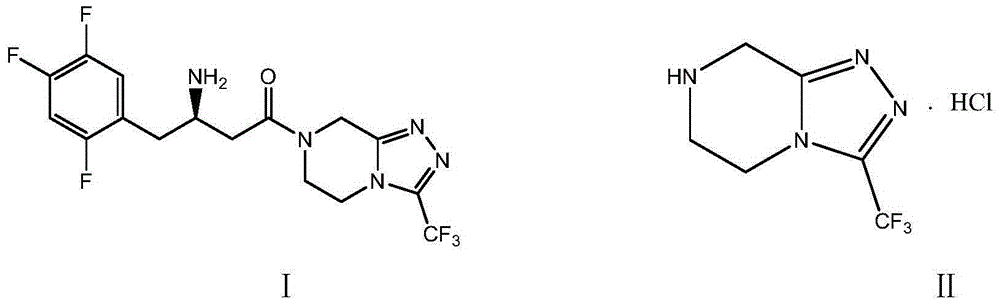
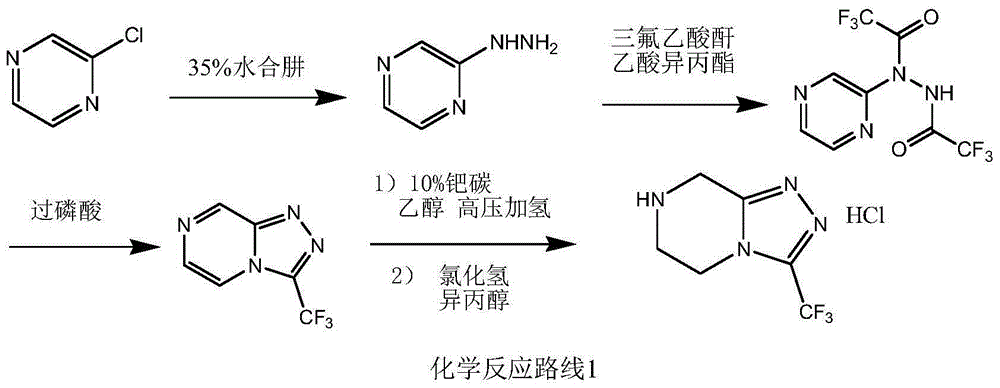
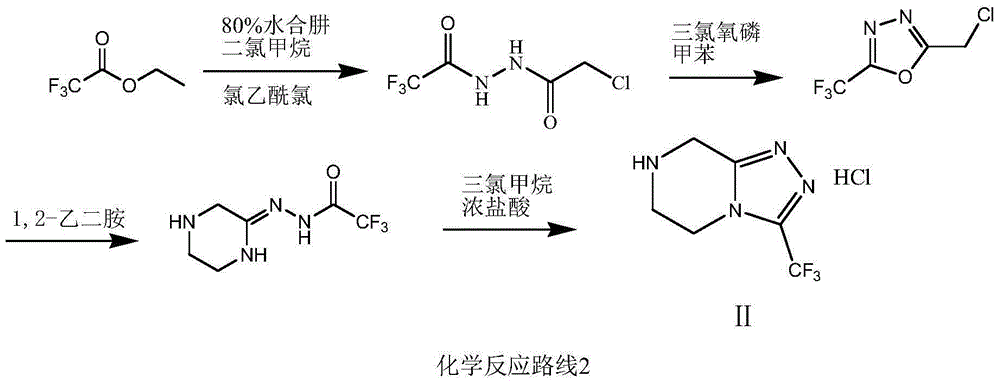
Preparation method of sitagliptin intermediate triazolopyrazine derivative
A technology of pyrazine hydrochloride and compound, applied in the direction of organic chemistry and the like, can solve the problems of high odor, high safety hazard, poor reaction selectivity, etc., and achieve the effects of simple process operation, short process flow and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Preparation of 3-trifluoromethyl-5,6,7,8-tetrahydro-1,2,4-triazolo[4,3-a]pyrazine hydrochloride (II)
[0052] In 250 milliliters of four-necked flasks, add successively 50 gram ethanols, 100 gram toluene, 17 gram sodium carbonate powders, 11.6 gram (0.1 moles) 2-chloroethylamine hydrochloride, 14.5 grams (0.10 moles) ethyl trifluoroacetate, After stirring and reacting at 30-35°C for 2 hours, add 14.5 grams (0.10 moles) of glycine ethyl ester hydrochloride, react at 50-55°C for 2 hours, stir and react at 90-95°C for 3 hours, and recover steam at the same time. ethanol out. Cool to 20-25°C, filter, wash the filter cake with 30 grams of toluene; transfer the combined filtrate to a 250 ml four-neck flask with a water separator, add 6.5 grams of 80% hydrazine hydrate dropwise between 20-25°C After about 1 hour, the dripping was completed, then stirred and reacted between 50-55°C for 2 hours, added 15 grams of hydrochloric acid with a concentration of 35%, refluxe...
Embodiment 2
[0057] Example 2: Preparation of 3-trifluoromethyl-5,6,7,8-tetrahydro-1,2,4-triazolo[4,3-a]pyrazine hydrochloride (II)
[0058] In 250 milliliters of four-necked flasks, add 50 gram methanol successively, 100 gram toluene, 17 gram sodium carbonate powders, 11.6 gram (0.1 mole) 2-chloroethylamine hydrochloride, 13.0 gram (0.1 mole) methyl trifluoroacetate, After stirring and reacting at 30-35°C for 2 hours, add 13.1 grams (0.10 moles) of glycine methyl ester hydrochloride, react at 50-55°C for 2 hours, stir and react at 90-95°C for 3 hours, and recover steam at the same time. Methanol out. Cool to 20-25°C, filter, and wash the filter cake with 30 grams of toluene; transfer the combined filtrate to a 250-ml four-neck flask with a water separator, and add 6.5 grams of 80% hydrated water dropwise between 20-25°C Hydrazine, about 1 hour after the drop, then stirred at 50-55 ° C for 2 hours, added 15 grams of 35% hydrochloric acid, refluxed azeotropic water removal, until the water...
Embodiment 3
[0059] Example 3: Preparation of 3-trifluoromethyl-5,6,7,8-tetrahydro-1,2,4-triazolo[4,3-a]pyrazine hydrochloride (II)
[0060] Replace 17 grams of sodium carbonate powder of embodiment 1 with 22 grams of potassium carbonate powder, all the other are the same as embodiment 1, obtain white solid 3-trifluoromethyl-5,6,7,8-tetrahydro-1,2,4- Triazolo[4,3-a]pyrazine hydrochloride (II) 19.1 g, yield 83.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com