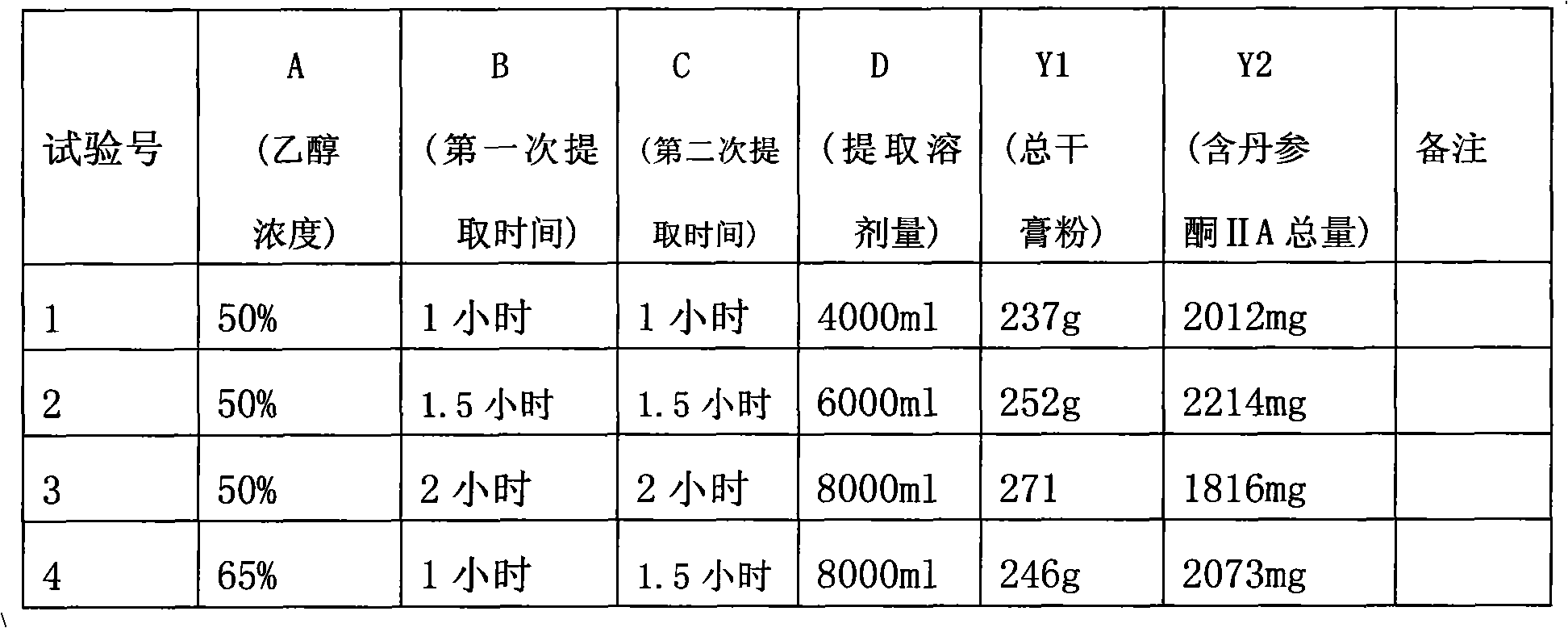
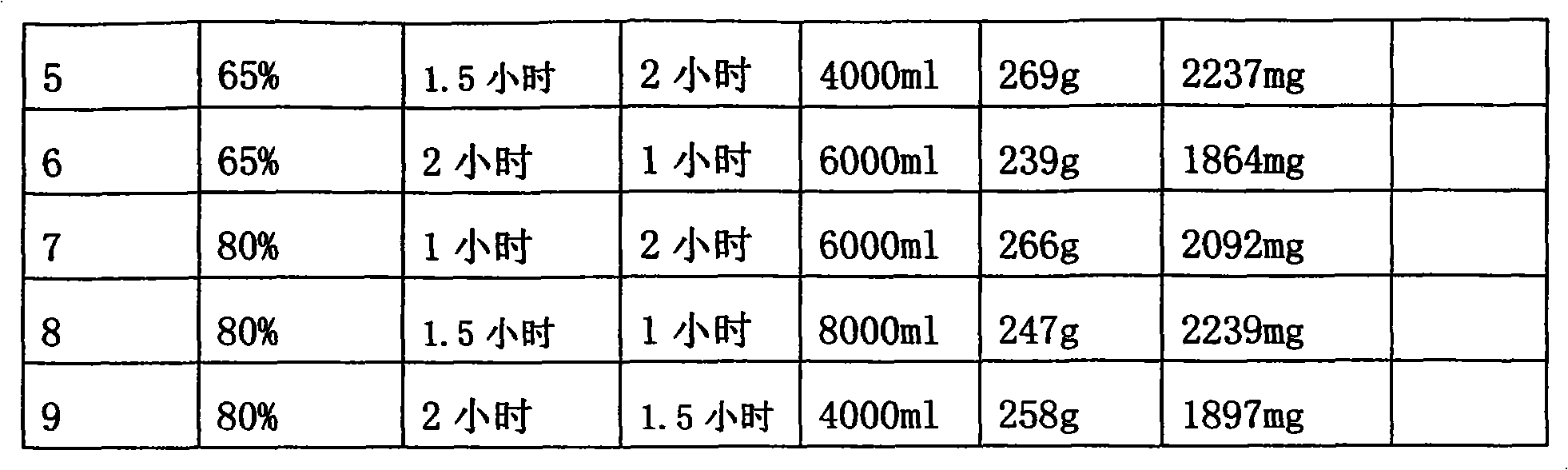
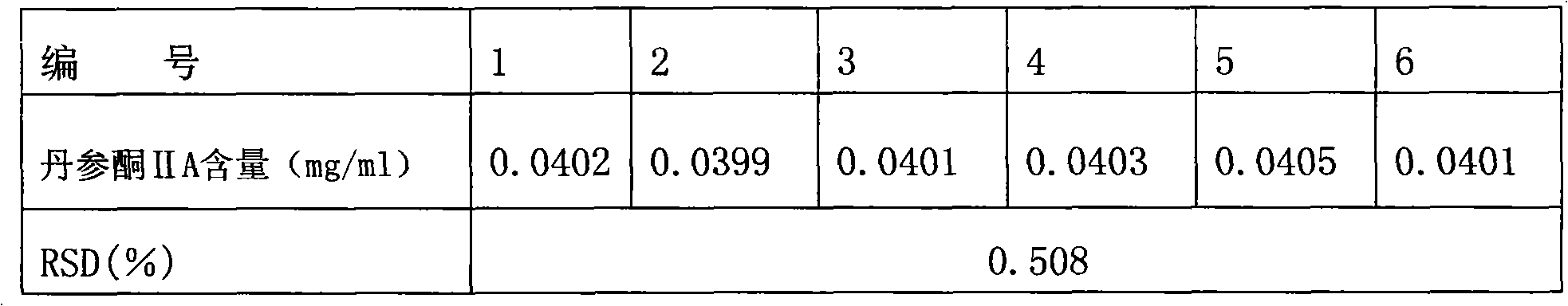
Preparation method of particles in formula of salvia miltiorrhiza bunge and quality detection method thereof
A technology of Danshen formula granules and a detection method, applied in the directions of pharmaceutical formulas, medical preparations containing active ingredients, measuring devices, etc., can solve the problem that the active ingredients cannot be guaranteed correspondingly, the properties are unstable, and it is difficult to ensure the safety and effectiveness of medicines for the masses. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] Example 1: Preparation method 1 of Danshen formula granules
[0185] Take 1000 grams of dried Salvia miltiorrhiza with no impurities, pulverize it into coarse powder with a pulverizer, put the Danshen coarse powder into an extraction tank, add 4000ml of ethanol with a concentration of 70%, heat to boiling, reflux for extraction for 1.5 hours, and collect the extract; The extract is filtered, and the filtrate is decompressed to recover ethanol at 55-65°C. After the ethanol is recovered, it can be spray-dried at about 85°C, and the obtained paste powder is collected for use; add water to the residual medicine residue in the extraction tank and heat it to boiling , Decoct for 1.5 hours, collect the decoction, filter, take the filtrate, concentrate under reduced pressure at 75~85℃ to a relative density of 1.03-1.20, spray dry at about 95℃, collect the obtained paste powder, and use it twice; For the prepared paste powder, add sucrose powder (or lactose powder) 1.5 times the to...
Embodiment 2
[0186] Example 2: Preparation method two of Danshen formula granules
[0187] Take 1000 grams of dried Salvia miltiorrhiza without impurities, pulverize it with a pulverizer into coarse powder, put the Salvia miltiorrhiza coarse powder into an extraction tank, add 4500ml of ethanol with a concentration of 75%, heat to boiling, reflux for extraction for 1.2 hours, and collect the extract; The extract is filtered. The filtrate is decompressed to recover ethanol at 55-60°C. After the ethanol is recovered, it can be spray-dried at about 85°C, and the obtained paste powder is collected for use; add water to the residual medicine residue in the extraction tank and heat it to boiling , Decoct for 1.2 hours, collect the decoction, filter, take the filtrate, concentrate under reduced pressure at about 80°C to a relative density of 1.05~1.10, spray dry at about 92°C, collect the obtained paste powder, and set aside; take twice to prepare Add sucrose powder (or soluble starch) with a weight...
Embodiment 3
[0188] Example 3: Detection method 1 of Danshen formula granules
[0189] Characters: should be yellow-brown to tan granules;
[0190] Ingredient identification:
[0191] Take 2g of this product, grind it finely, add 5ml of ether, shake, place for 1 hour, filter, evaporate the filtrate to dryness, add 1ml of ethyl acetate to the residue to dissolve it, as the test solution. Take 1g of Salvia miltiorrhiza reference medicinal material, and prepare the reference medicinal material solution in the same way. According to the Chinese Pharmacopoeia 2005 edition, an appendix VIB thin layer chromatography, draw 5μl of each of the above two solutions, and pour them on the same silica gel G thin layer plate. Ethyl ester = 19:1 is the developing agent, unfold, take out, and dry. In the chromatogram of the test product, spots of the same color appear on the corresponding position of the chromatogram of the control medicinal material;
[0192] Inspection: It should comply with the relevant regulat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com