Radical therapeutic agent for keloid and hypertrophic scar
A technology for hypertrophic scars and keloids, which is applied in the field of elastic fiber formation accelerators and radical therapeutic drugs for keloids and/or hypertrophic scars, and can solve problems such as the inability to develop radical therapeutic effects on keloids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0098] Hereinafter, the present invention will be described in more detail based on examples, but the present invention is not limited by these examples.
reference example 1
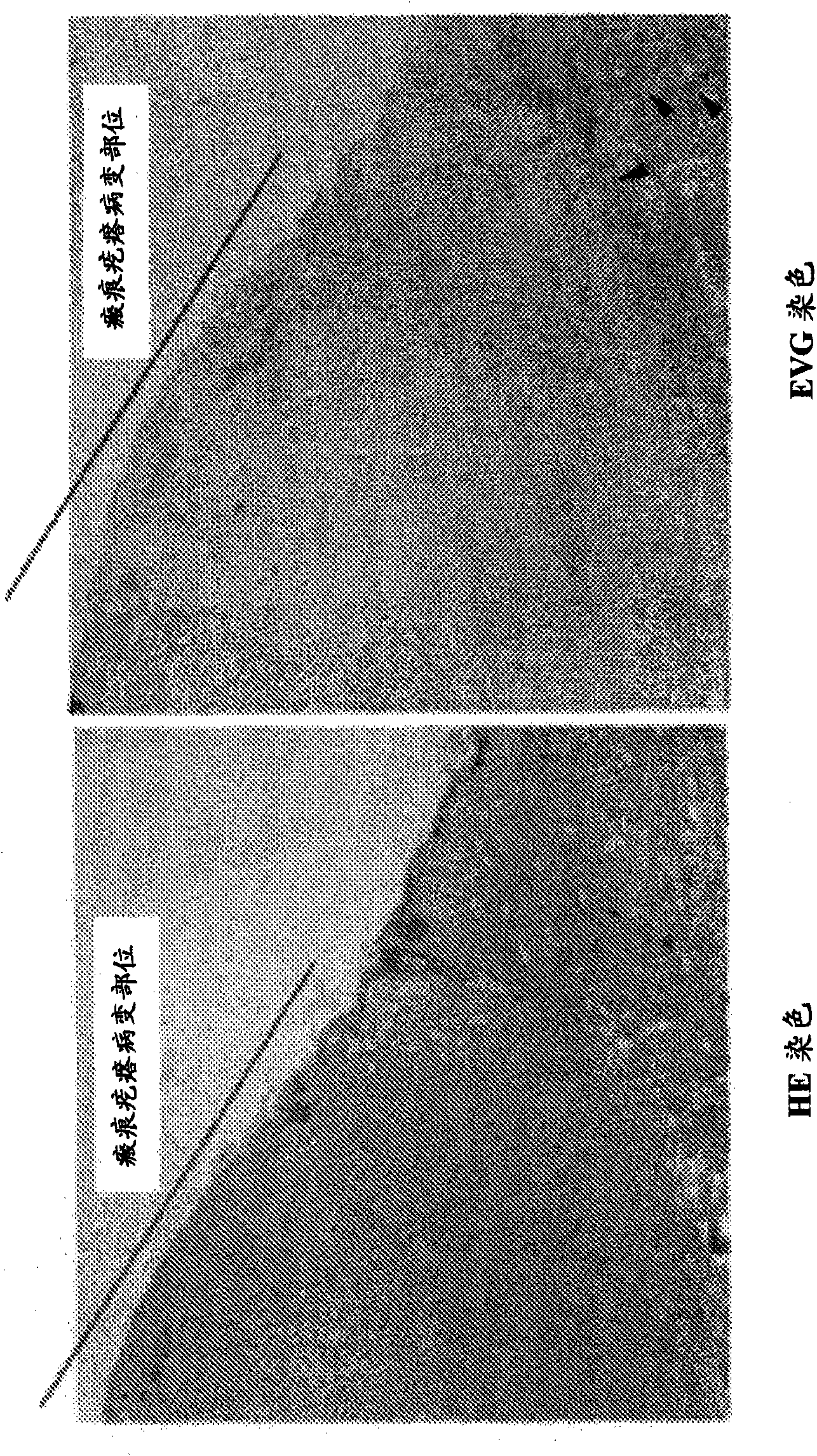
[0099] [Reference Example 1] Observation of keloid lesion site and normal skin site
[0100] Human tissue samples containing keloid lesions and normal skin parts were removed from keloid patients as tissue materials. The samples were fixed with 4% paraformaldehyde at 4°C for 24 hours, and then embedded in paraffin to prepare paraffin blocks and 3 μm paraffin sections. After deparaffinization, hematoxylin-eosin (HE) staining and elastic van Gieson (EVG) staining were performed to prepare the specimens. A microscope is used to look at the keloid tissue and normal skin tissue.
reference example 2
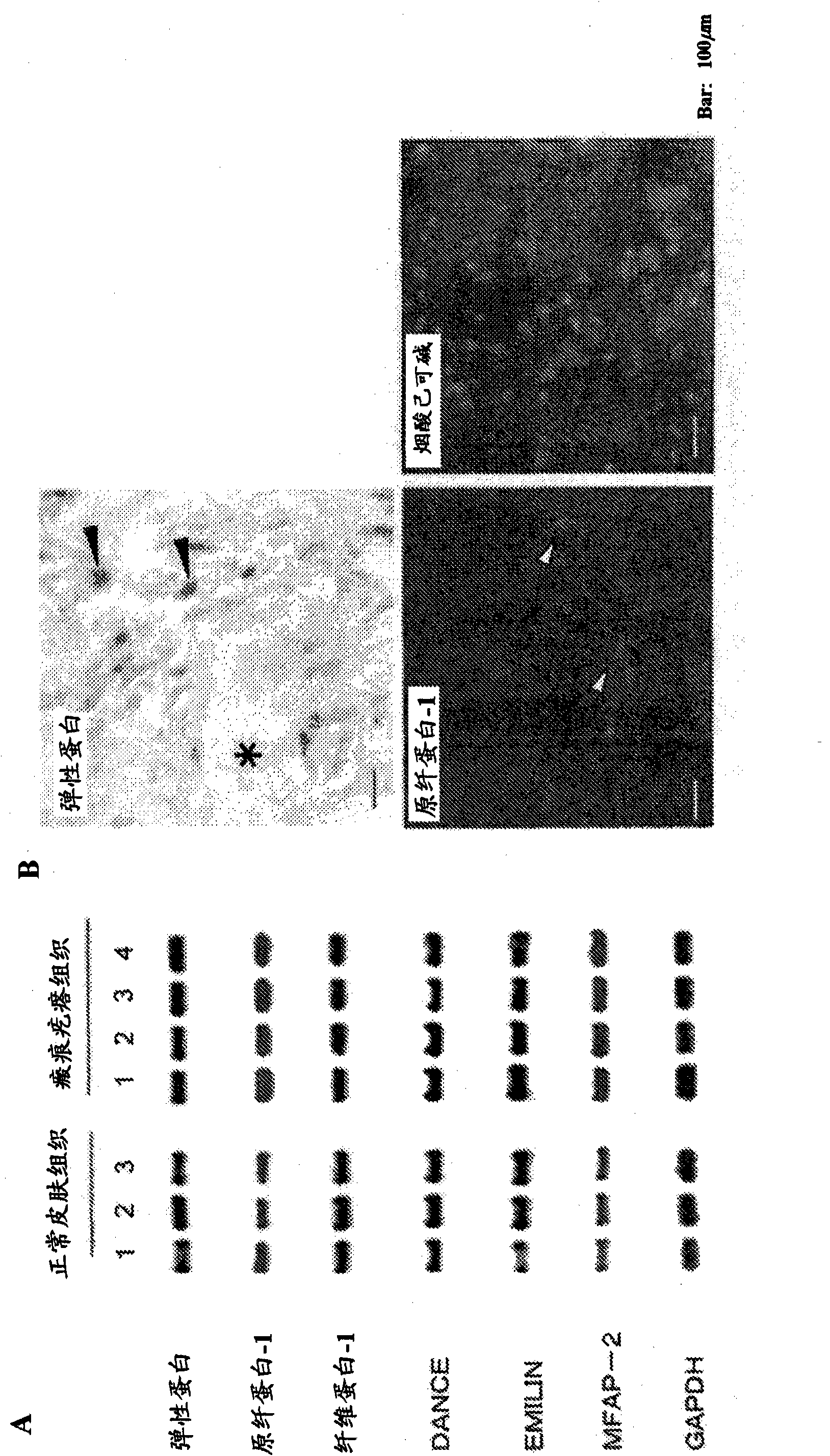
[0101] The result is as figure 1 shown. The part marked with a line is the keloid lesion site, and the adjacent part is the normal skin site. By EVG staining, the elastic fiber structure stained black (arrow) can be confirmed in the normal skin site other than the keloid lesion site, and the majority of the keloid lesion site can be seen to be transparent, and the lack of elastic fiber structure formation can be confirmed. [Reference Example 2] mRNA Expression of Elastic Fiber Components in Keloid Lesion Tissue and Normal Skin Tissue
[0102] Keloid lesions (4 persons) and normal skin tissues (3 persons) were removed from human samples as tissue materials. Total RNA was extracted from keloid tissue and normal skin tissue using RNeasy Plus kit (manufactured by Keygen Co., Ltd.). cDNA was synthesized from 1 μg of total RNA using an advantage RT for PCR kit (manufactured by Nippon Beckton Ditsukinn Co., Ltd.), and the expression of mRNA of seven proteins constituting elastic f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com