In-situ gel composition for eyes
An in-situ gel and ophthalmic technology, which is applied in the direction of non-active ingredient medical preparations, active ingredient-containing medical preparations, peptide/protein components, etc., can solve the problem of ophthalmic in-situ gel without nerve growth factor And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
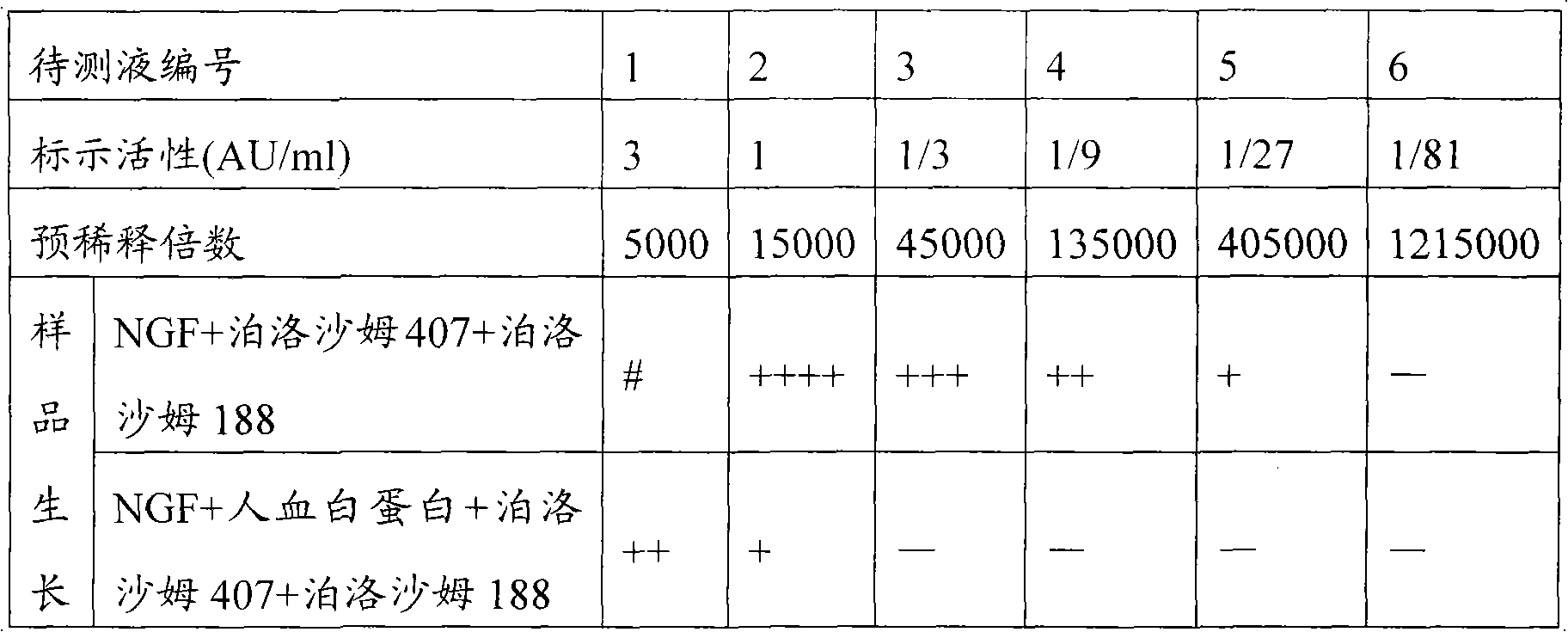
[0062] The proportioning ratio of this embodiment is shown in the following table:
[0063] NGF
[0064] Preparation method: Take an appropriate amount of water for injection, add gellan gum dispersedly while stirring, stir at 90°C to completely dissolve the gellan gum to form a transparent solution, and cool to room temperature. NGF, alanine, glycine, arginine, and benzyl alcohol are first dissolved in an appropriate amount of water for injection, and after sterilization and filtration, they are added to the gellan gum solution prepared above, and the water for injection is supplemented to a sufficient amount, stirred evenly, and sterile Subpackage.
Embodiment 2
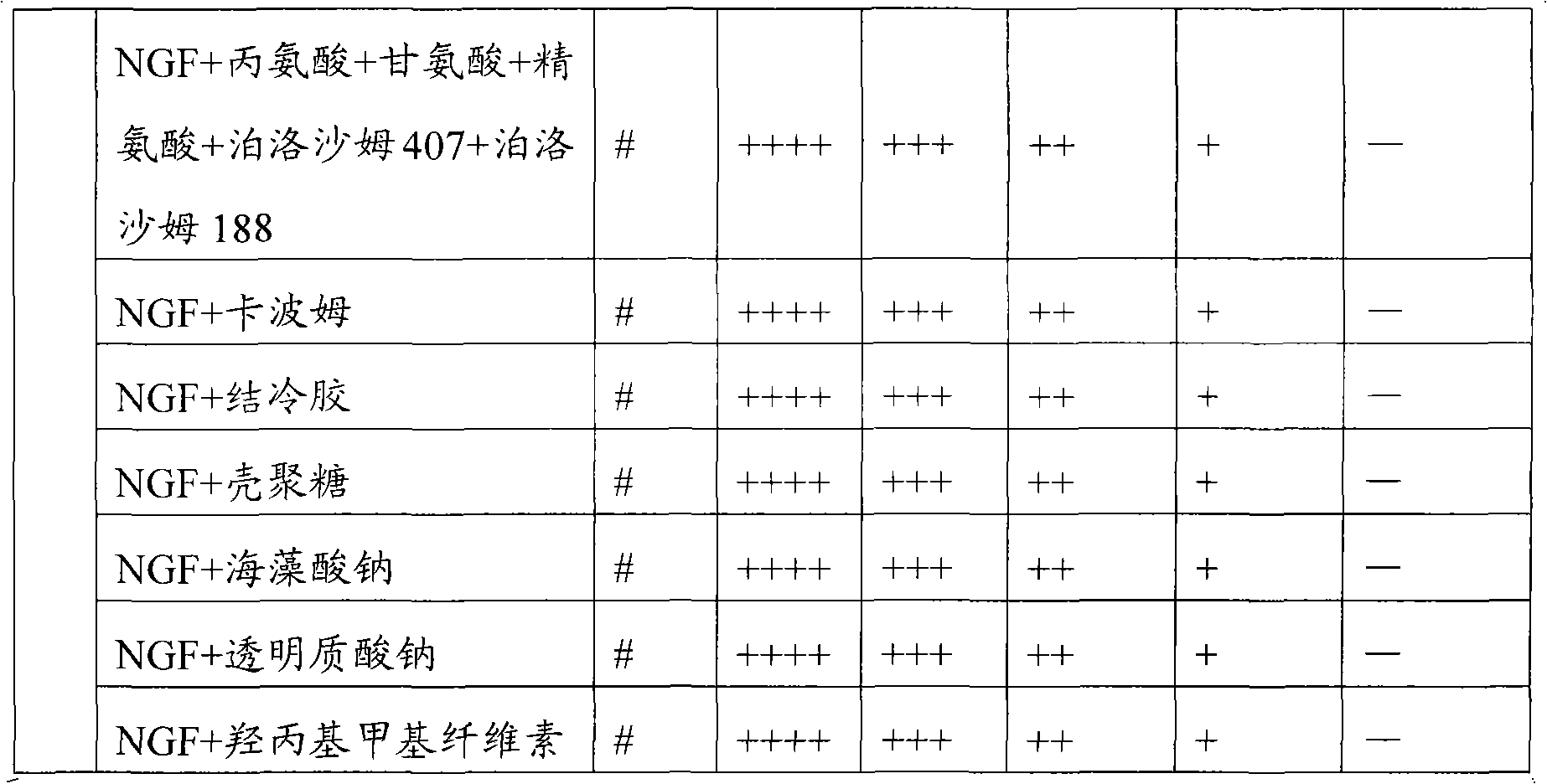
[0066] The proportioning ratio of this embodiment is shown in the following table:
[0067] NGF
200μg / ml
3.3mg / ml
3.3mg / ml
3.3mg / ml
gellan gum
4mg / ml
9mg / ml
acetate buffer
Adjust to pH5.5
50mg / ml
Water for Injection
Appropriate amount
[0068] Preparation method: Take an appropriate amount of water for injection, add gellan gum and benzyl alcohol dispersedly while stirring, stir at 90°C to completely dissolve the gellan gum to form a transparent solution, and cool to room temperature. Dissolve NGF, alanine, glycine, arginine and mannitol in pH 5..5 acetate buffer solution, sterilize and filter, and freeze-dry by low-temperature freeze-drying method. Just before use, the gellan gum solution was added to the NGF freeze-dried solution, dissolved and mixed evenly.
[0069] The above-mentioned gellan gum is a microbial exopoly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com