Chiral oxidation catalyst and preparation method thereof
An oxidation catalyst, chiral technology, applied in physical/chemical process catalysts, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve problems such as enantiomeric drug pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
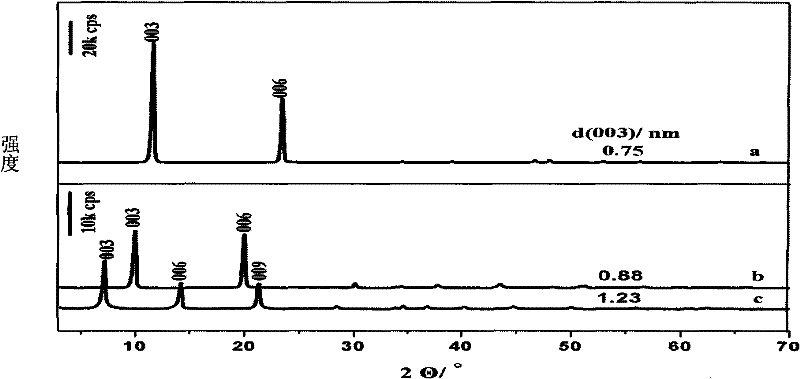
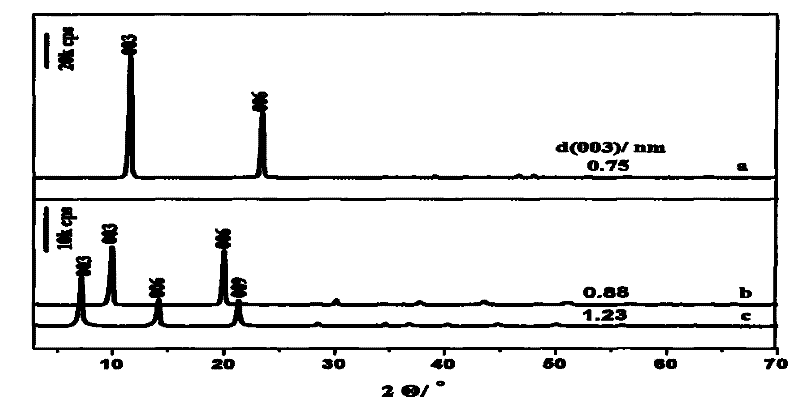
[0021] A. Add 0.01mol zinc nitrate hexahydrate, 0.005mol aluminum nitrate nonahydrate and 0.035mol urea into 320ml deionized water, dissolve and transfer to a 500ml three-necked flask, stir and heat to reflux for 24 hours. After the reaction, the product was suction filtered, washed with deionized water until the pH was 7, then washed once with ethanol to disperse the product evenly, and the filter cake was dried in an oven at 60°C to obtain a solid powder, which was Zn-Al-CO 3 -LDHs
[0022] B. Will get Zn-Al-CO 3 0.4g of LDHs was added to NaNO dissolved in 1.5mol / L 3 solution, add 0.002mol concentrated HNO 3 , N 2 protection, stirred and crystallized at room temperature for 24 hours, after the reaction, the product was suction filtered, washed with deionized water until the pH was 7, then washed once with ethanol to make the product evenly dispersed, and the filter cake was dried in an oven at 25°C to obtain a solid powder, namely Zn-Al-NO 3 - LDHs precursors.
[0023]...
Embodiment 2
[0027] A. take step A method in the similar embodiment 1 to obtain Zn-Al-CO 3 -LDHs.
[0028] B. Obtain Zn-Al-NO by the method of step B among the similar embodiment 1 3 -LDHs precursor
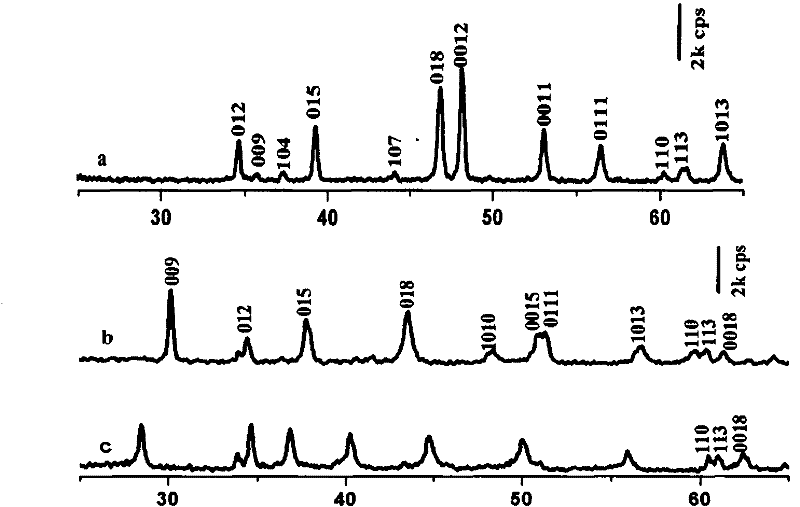
[0029] C. the pH value of L-glutamic acid is adjusted to 12 with the NaOH solution of 2mol / l, and preparation concentration is the negatively charged L-glutamic acid anion solution of 0.086mol / l, adds hydrotalcite precursor 0.15g, in 60℃ in N 2 The reaction was carried out for 12h under protection and stirring, and the product was used to remove CO 2 Washing with water, fully washing with absolute ethanol water, centrifuging, and vacuum drying at 25°C to obtain intercalated L-glutamic acid intercalated hydrotalcite, and then stored under dry conditions.
[0030] D. Add 50μl VO(O-i-Pr) 3 Add 1ml of dichloromethane to obtain a solution with a concentration of 0.5mol / L, then add the L-glutamic acid intercalation hydrotalcite prepared by C to the above solution, stir at 40°C for 1 hour, and ...
Embodiment 3
[0033] A. take step A method in the similar embodiment 1 to obtain Zn-Al-CO 3 -LDHs.
[0034] B. Obtain Zn-Al-NO by the method of step B among the similar embodiment 1 3 -LDHs precursor
[0035] C. Adjust the pH value of L-glutamic acid to 10 with concentrated ammonia water, and prepare a negatively charged L-glutamic acid anion solution with a concentration of 0.3mol / l, add 0.15g of hydrotalcite precursor, and put it under N at 20°C 2 The reaction was carried out for 48h under protection and stirring, and the product was used to remove CO 2 Washing with water, fully washing with absolute ethanol water, centrifuging, and vacuum drying at 25°C to obtain intercalated L-glutamic acid intercalated hydrotalcite, and then stored under dry conditions.
[0036] D. Add 25μl VO(O-i-Pr) 3 Add 1ml of dichloromethane to obtain a solution with a concentration of 0.1mol / L, then add the L-glutamic acid intercalation hydrotalcite prepared by C to the above solution, and stir at 10°C for 5h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com