New preparation method for crystal form Gefitinib Form 1
A technology of crystal form and ethanol, applied in the field of polymorphic drug preparation, can solve the problems of increased operating cost, uncontrollability, hidden dangers in process amplification, etc., and achieves low cost, good crystallinity of crystal form, increased controllability and The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
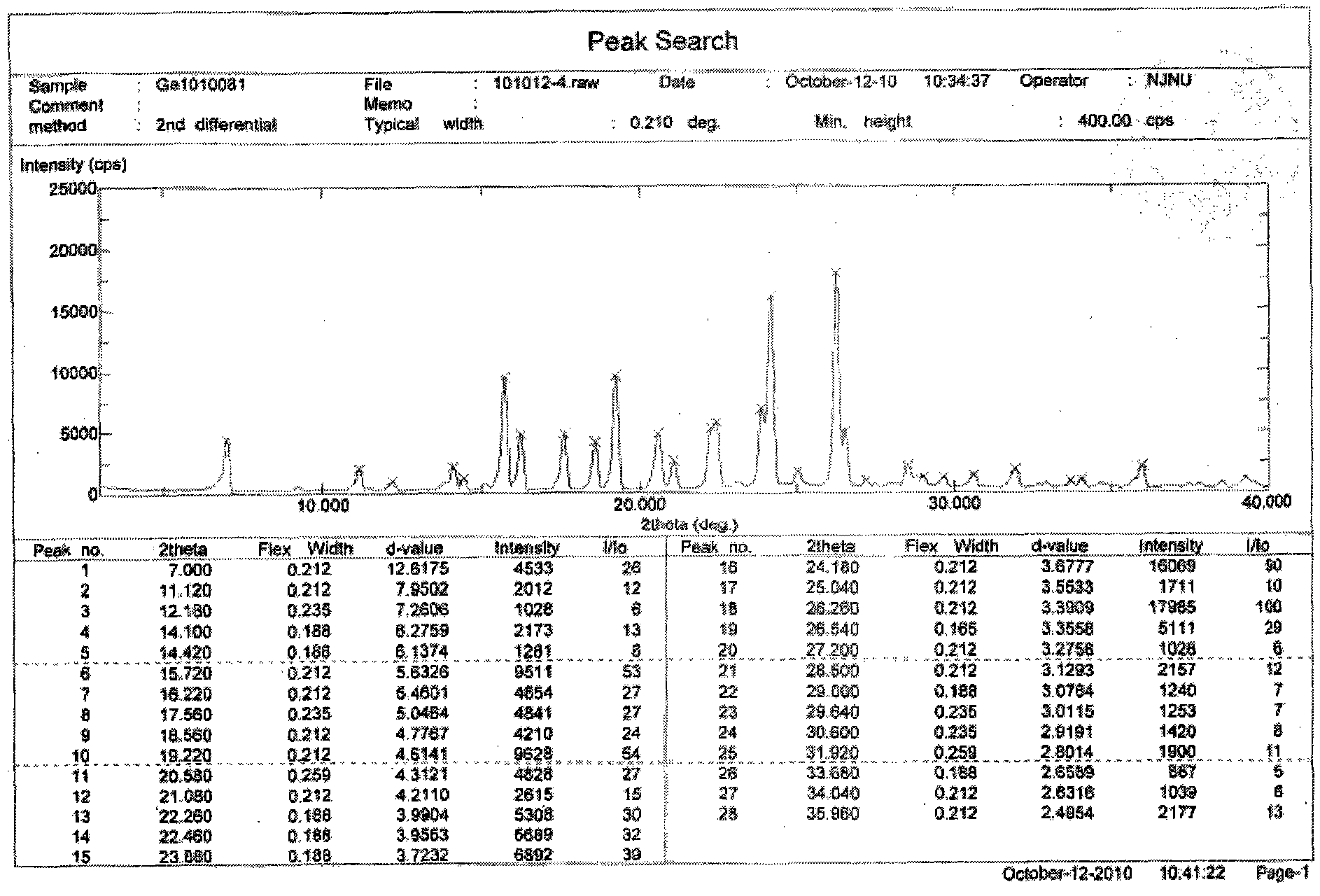
[0023] Embodiment 1: Preparation of Gefitinib Form 1 crystal form
[0024] Weigh Gefitinib (1g), then add it to a reaction flask, add 20mL of ethanol, control the temperature at 75°C, stir magnetically, slowly cool down to room temperature after dissolving, filter, wash with cold ethanol (3×1mL), and wash at 40°C After vacuum drying, 900 mg of Gefitinib Form I crystal form was obtained with a yield of 90%.
Embodiment 2
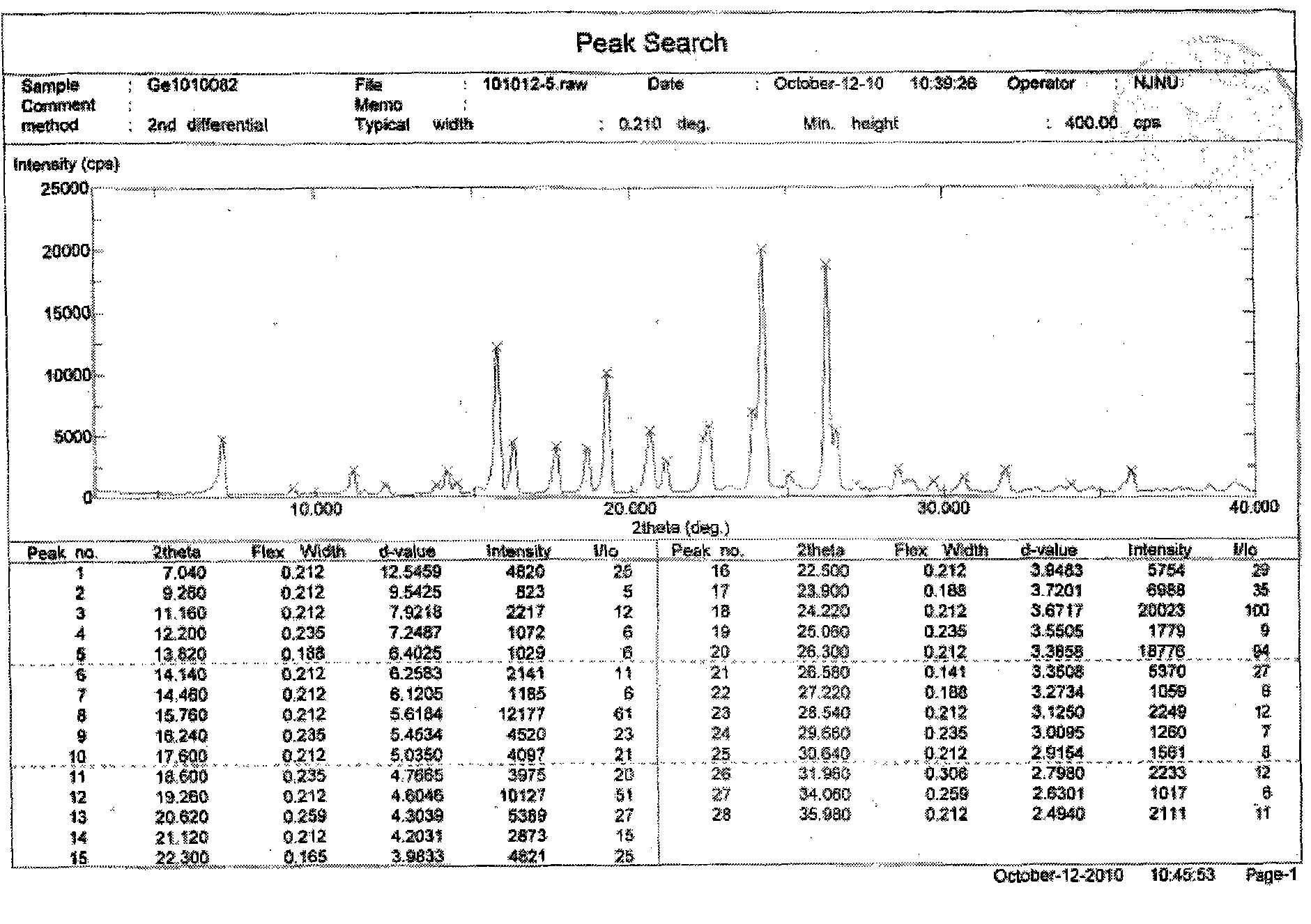
[0025] Embodiment 2: Preparation of Gefitinib Form 1 crystal form
[0026] Weigh Gefitinib (1g), then add it to the reaction flask, add 30mL isopropanol, control the temperature at 75°C, stir magnetically, slowly cool down to room temperature after dissolving, filter, and use cold isopropanol (3×1mL) After washing and vacuum drying at 40°C, 940 mg of Gefitinib Form I was obtained with a yield of 94%.
Embodiment 3
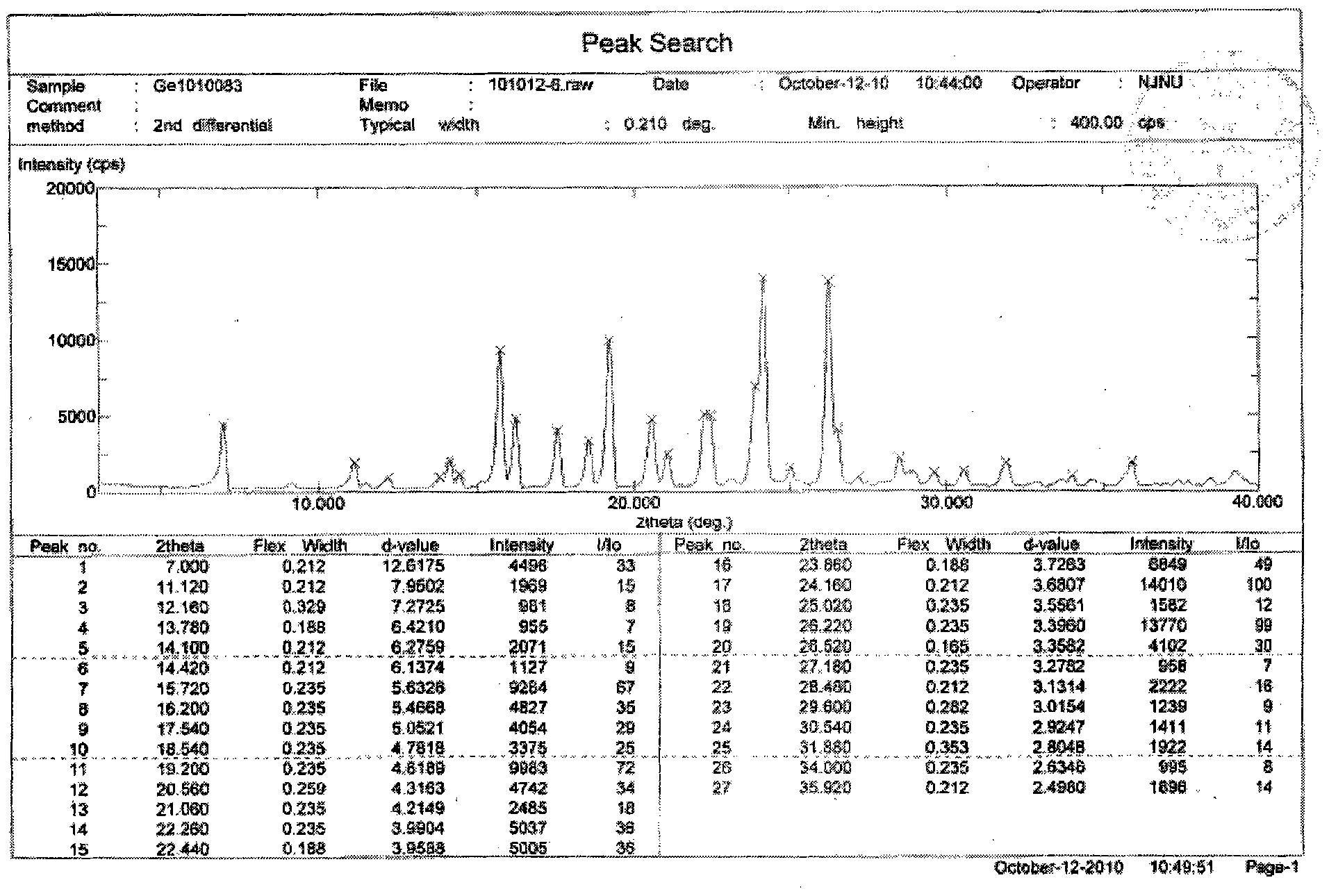
[0027] Embodiment 3: Preparation of Gefitinib Form 1 crystal form
[0028] Weigh Gefitinib (1g), then add it to the reaction flask, add 20mL n-butanol, control the temperature at 75°C, stir magnetically, slowly cool down to room temperature after dissolving, filter, and use cold n-butanol (3×1mL) After washing and vacuum drying at 40°C, 890mg of Gefitinib Form I was obtained with a yield of 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com