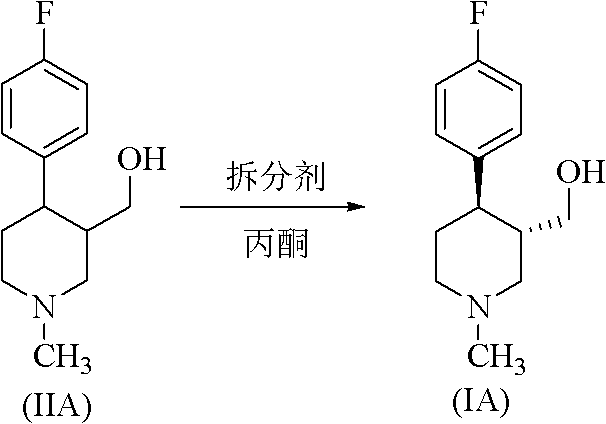
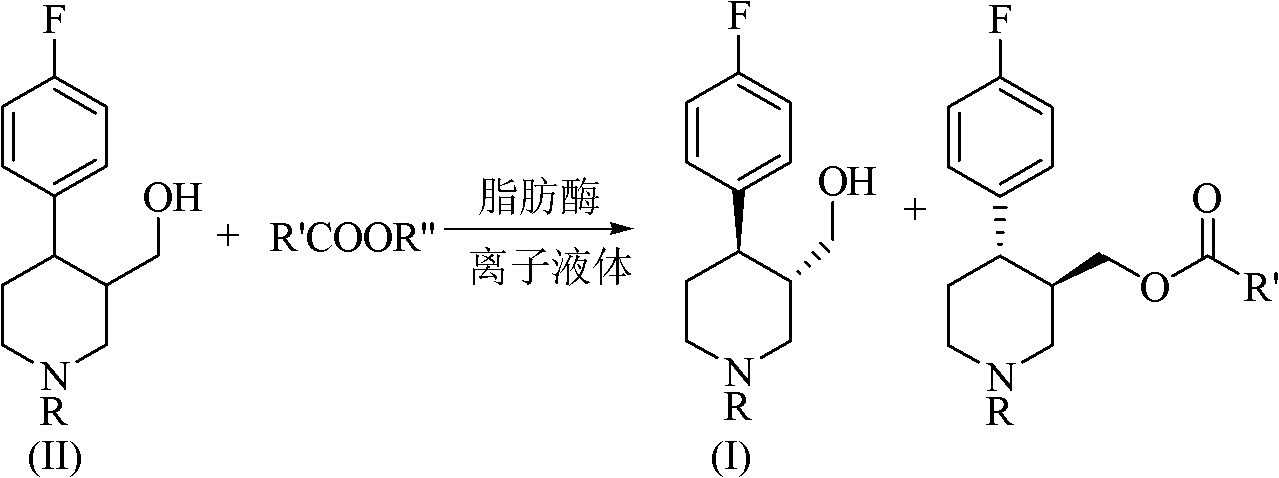
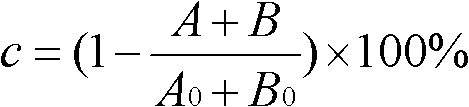Method for preparing paroxetine intermediate through enzymatic resolution in ionic liquid
A technology of ionic liquids and intermediates, applied in the field of biocatalysis, can solve the problems of potential danger in operation, environmental pollution, strong toxicity, etc., and achieve the effects of less environmental pollution, easy separation of products, and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add the substrate compound (IIA) at a concentration of 30mmol / L, use the ionic liquid [BMIm][PF6] as the reaction medium, add the acyl donor according to the compound (IIA):vinyl acetate ratio of 1:10, add 5g / L lipase from Porcine pancreatic was used to start the reaction, and the reaction was carried out at 30°C and the shaker speed was 200rpm for 12h. After the reaction, the lipase was removed by centrifugation to obtain a supernatant. The supernatant was extracted with a mixed solution of n-hexane / isopropanol (90:10, v / v) to obtain an organic phase, which was filtered and analyzed by HPLC. The reaction conversion rate was 38%, and the optical purity was 79%.
Embodiment 2
[0041] Add the substrate compound (IIA) with a concentration of 30mmol / L, use the ionic liquid [BMIm][PF6] as the reaction medium, add the acyl donor according to the ratio of compound (IIA):vinyl acetate in the amount of 1:10, add 5g / L lipase A from Candidaantarctica was used to start the reaction, and the reaction was carried out at 30°C and the shaker speed was 200rpm for 10h. After the reaction, the lipase was removed by centrifugation to obtain a supernatant. The supernatant was extracted with a mixed solution of n-hexane / isopropanol (90:10, v / v) to obtain an organic phase, which was filtered and analyzed by HPLC. The reaction conversion rate was 52%, and the optical purity was 92%.
Embodiment 3
[0043] Add the substrate compound (IIA) with a concentration of 30mmol / L, use the ionic liquid [BMIm][PF6] as the reaction medium, add the acyl donor according to the ratio of compound (IIA):vinyl acetate in the amount of 1:10, add 5g / L lipase B from Candidaantarctica was used to start the reaction, and the reaction was carried out at 30°C and the shaker speed was 200rpm for 8h. After the reaction, the lipase was removed by centrifugation to obtain a supernatant. The supernatant was extracted with a mixed solution of n-hexane / isopropanol (90:10, v / v) to obtain an organic phase, which was filtered and analyzed by HPLC. The reaction conversion rate was 46%, and the optical purity was 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com