Process for preparing a pharmaceutical formulation of contrast agents
A preparation and drug technology, applied in the field of nuclear magnetic resonance imaging and chelate complexes, can solve complex tolerance problems, the need for perfect control rates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0189] 1) Example 1: Tolerance in vivo
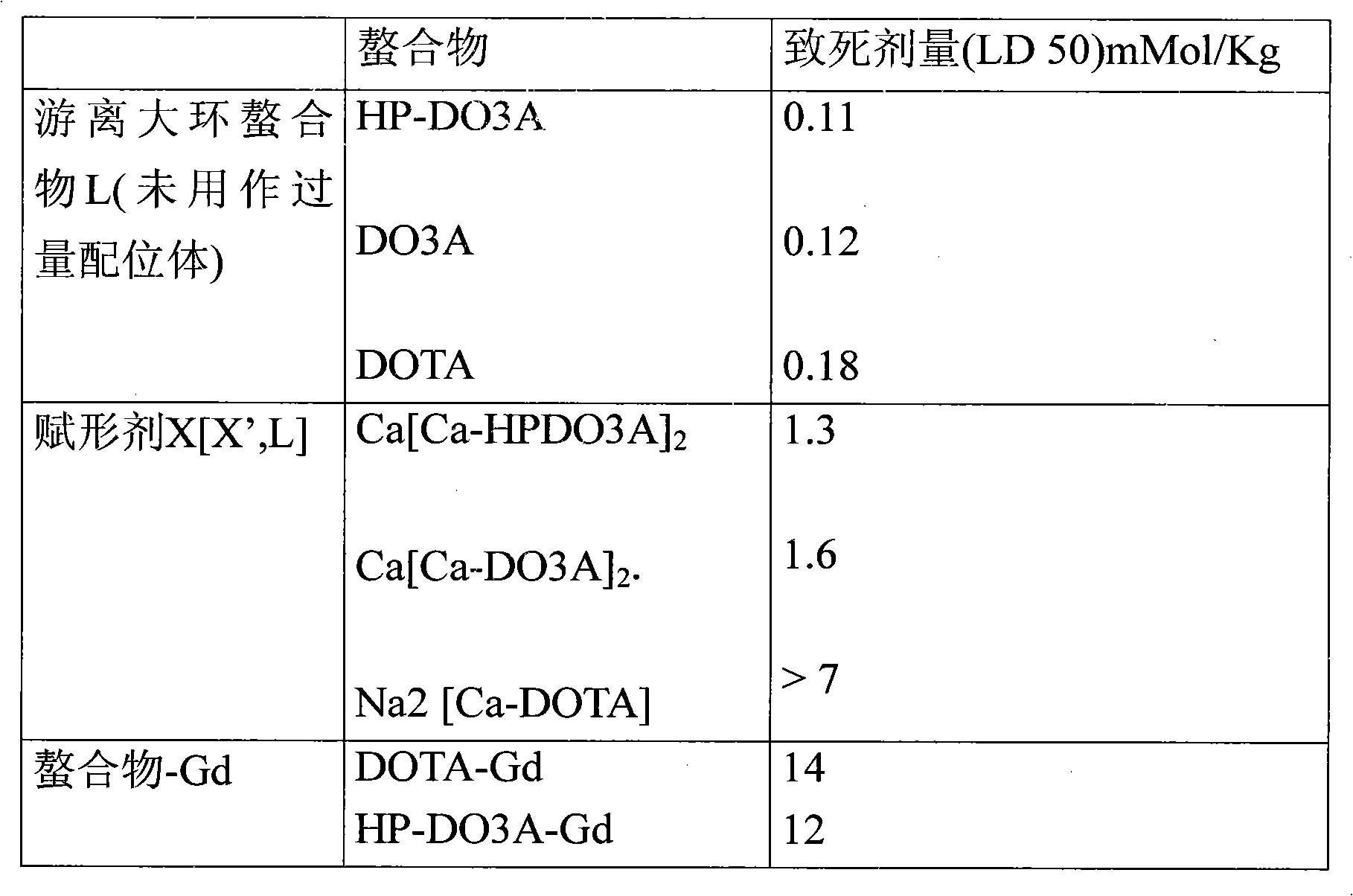
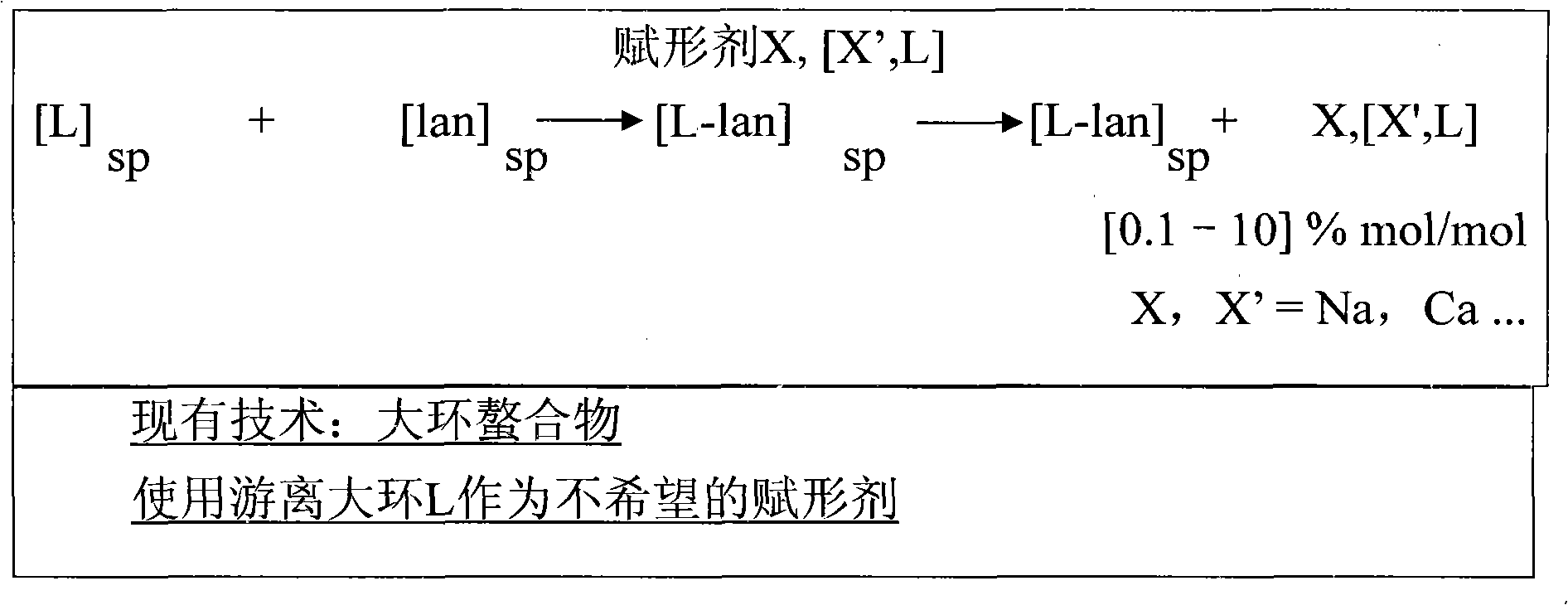
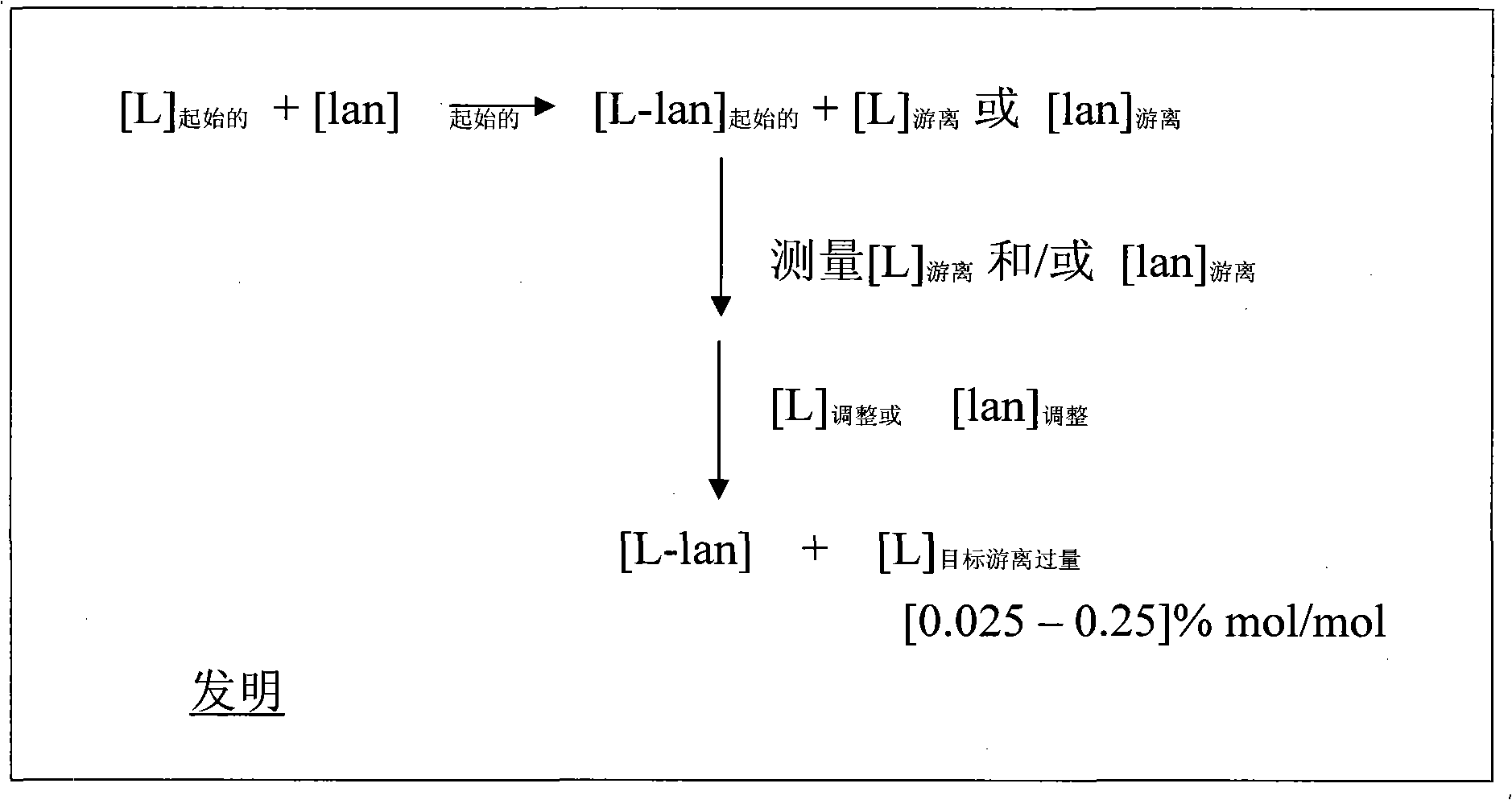
[0190] Tolerability results in Table 2 (acute toxicity in mice for DOTA diagnostic solution; this solution is an injected drug solution and contains DOTA with Gd 3+ complexes and not composed of Gd 3+ Excess free DOTA complexed and not complexed by metal ions as excipients) showed that formulations containing free macrocyclic chelate DOTA from 0.025 mol / mol% to 0.25 mol / mol% were three times less toxic at close to 2% of the formulation.
[0191]
[0192]
[0193] Additional stability studies carried out by the applicant showed that the formulations were very satisfactorily free of gadolinium release over long storage times.
Embodiment 2
[0194] Example 2: Process for the preparation of formulations of lanthanide chelates (mixture of chelate solution and lanthanide solution)
[0195] The preparation of formulations in which the macrocyclic chelate is DOTA is more precisely described. Table 3 below gives an example of the quantities (industrial quantities) used for the manufacture of 100 liters of DOTA solution.
[0196]
[0197] (1) 1,4,7,10-tetraazacyclododecane-N,N',N",N"'-tetraacetic acid
[0198] step 1 : dissolve
[0199] 40 liters of injection grade water was placed in a 100-liter manufacturing tank at 80°C, nitrogen injection was started, and then 20.100 kg of DOTA and 9.135 kg of gadolinia were mixed under agitation. Complexation is performed at a pH below 6, for example between 3 and 6, for example at pH 4. Gadolinium oxide forms a water-soluble acidic complex in the presence of DOTA.
[0200] step 2 :Measurement
[0201] After step 1, samples were taken and free gadolinium was determined...
Embodiment 3
[0214] 2) Example 3: Process for making formulations of lanthanide chelates (Dissolution of solid complexes [chelates-lanthanides])
[0215]
[0216] This example illustrates the manufacture of a small number of products, with appropriate exchange on an industrial scale.
[0217]
[0218] In a three-necked flask equipped with a condenser, a thermometer, and a pH meter, 10 g (0.025 mol; 1 equivalent) of the macrocyclic chelate DOTA was dissolved in 200 ml of water by heating to 80°C. The measured pH was 3.7. Adjust it to 6 with 2N NaOH solution. 4.48 g (0.0125 mol; 0.5 equivalents) of gadolinia were added. The pH was adjusted and kept stable between 6 and 7 by adding 1N HCl. The reaction was placed at 80 °C with stirring.
[0219] Residual free gadolinium was removed by means of a chelex resin prewashed with water. In order to do this, the reaction mixture was brought to pH 5 (resin is more efficient). The whole was left at room temperature for 2 hours with stirri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com