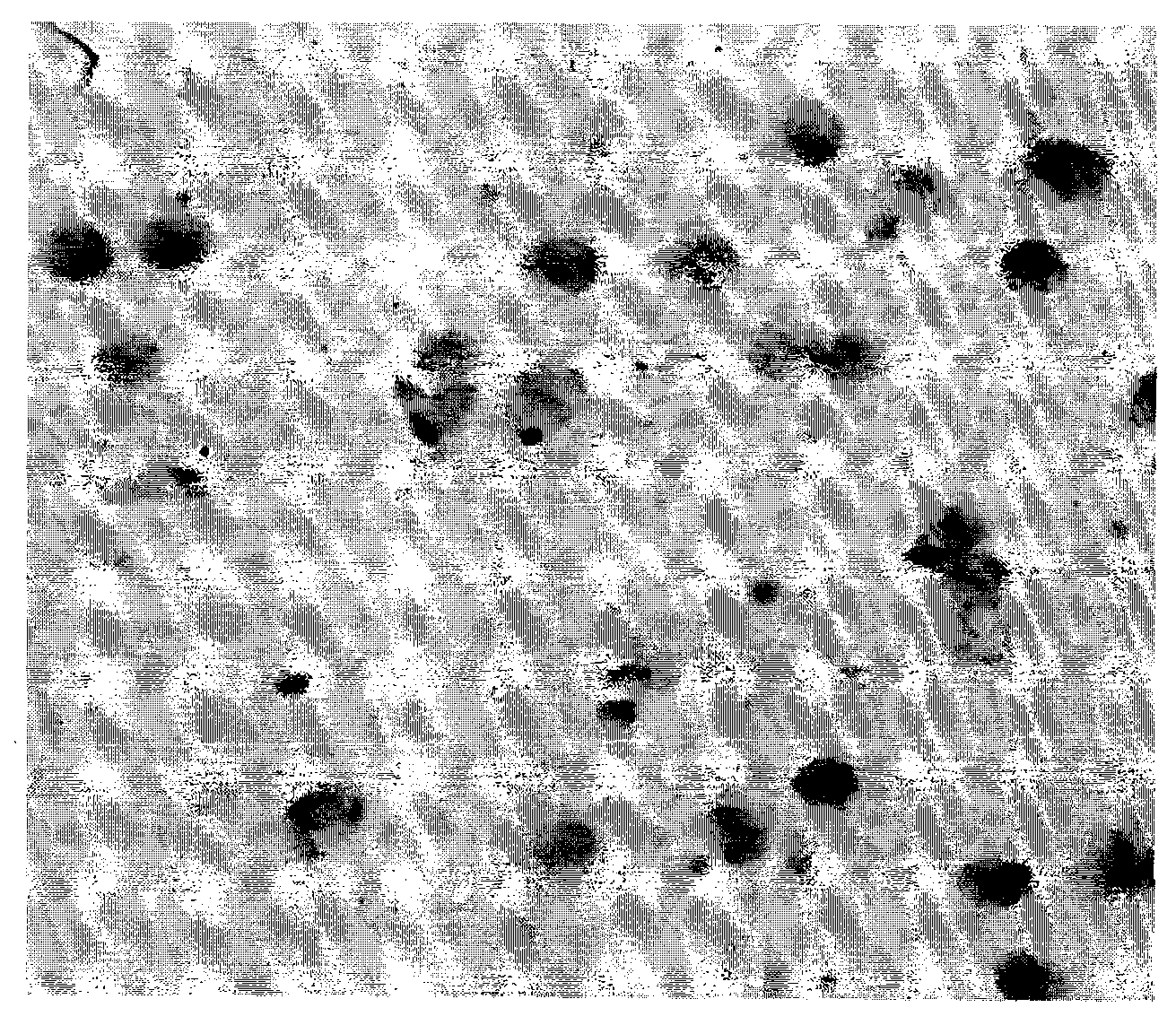
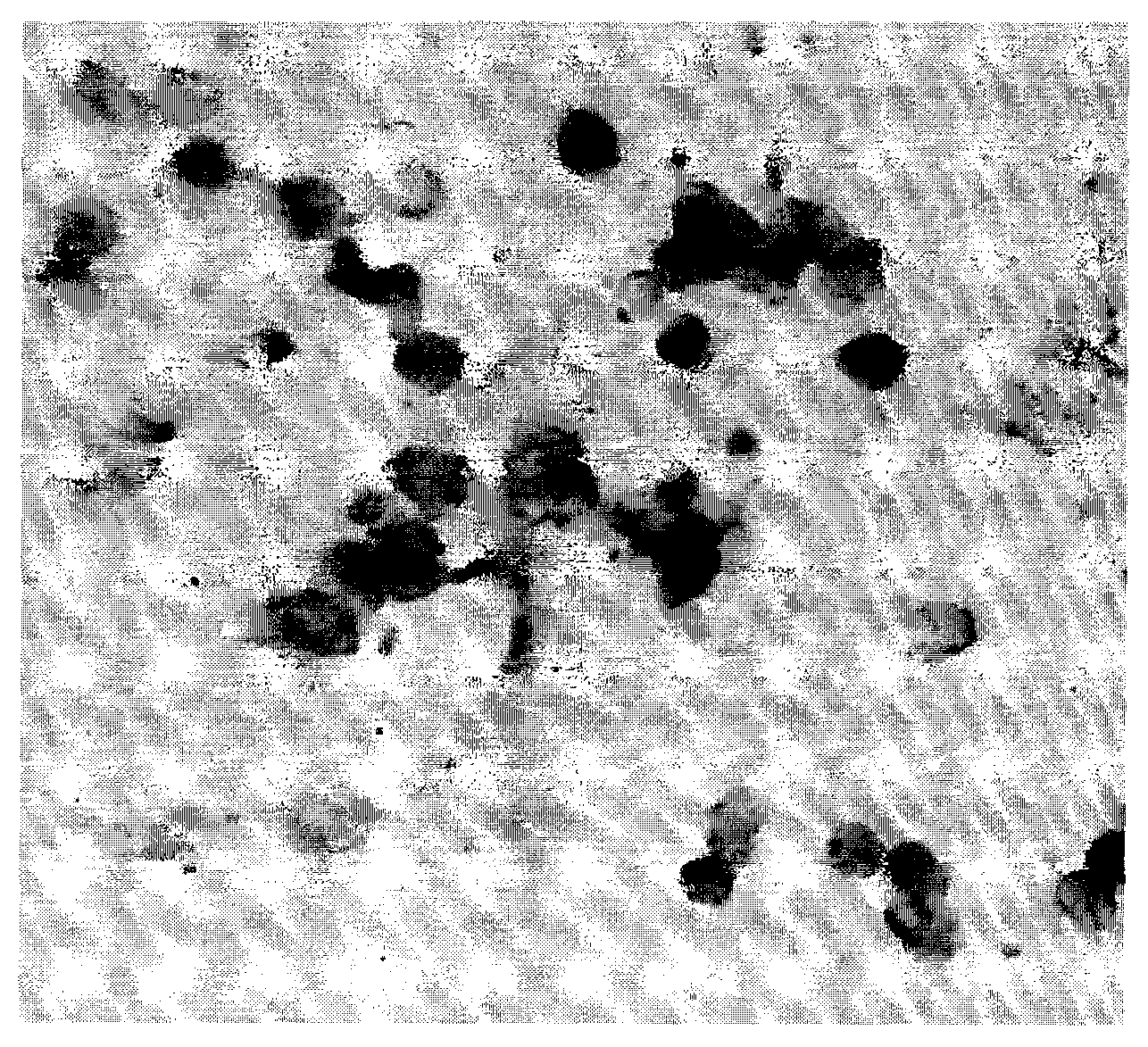In-situ hybridization detection kit for PD-ECGF genes and detection method and application thereof
A detection kit and in situ hybridization technology, applied in the field of kits, can solve the problems of heterogeneity, tumor cell drug resistance, failure of anti-cancer battle, etc., and achieve the effects of convenient operation, high sensitivity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] An in situ hybridization detection kit for PD-ECGF gene, comprising a hybridization probe, a marker, and a potentiator, wherein the sequence of the hybridization probe is shown in SEQ ID NO.1. Hybridization probes were labeled with digoxigenin. The composition of other liquids and specimens in the kit is as follows:
[0046] Digestive solution 100μl / tube 1 tube / box Colorless transparent liquid
[0047] Protective solution 100μl / tube 1 tube / box Colorless transparent liquid
[0048] Pre-hybridization solution 1300μl / tube 2 tubes / box Colorless transparent liquid
[0049] Sense hybridization solution 10μl / tube 1 tube / box Colorless transparent liquid
[0050] Antisense hybridization solution 10μl / tube 1 tube / box Colorless transparent liquid
[0051] Blocking solution 1000μl / tube 1 tube / box Colorless transparent liquid
[0052] Alkaline phosphatase antibody 1μl / tube 1 tube / box Colorless transparent liquid
[0053] Chromogen A 175μl / tube 1 tube / box Yellow liquid
[0054] ...
Embodiment 2
[0096] A kind of PD-ECGF gene in situ hybridization detection method and its kit application
[0097] 1. Specimen processing
[0098] 1. Use a 10ml centrifuge tube to fill 4.5ml of lymphocyte separation solution, then slowly add 3ml of anticoagulated blood into the centrifuge tube containing lymphocyte separation solution (blood: lymphocyte separation solution = 1:1.5), and centrifuge at 2000r / min 10min;
[0099] 2. Take the white blood cells in the middle layer into another centrifuge tube, then add about twice the amount of 1× buffer I to this tube, mix well, and centrifuge at 1500g / min for 10min;
[0100] 3. Discard the supernatant. Add about twice the 1× buffer I to the precipitate, mix well, and centrifuge at 1500g / min for 10min;
[0101] 4. Discard the supernatant, and absorb the excess liquid from the mouth of the test tube with paper towels. Then the precipitate was made into a suspension, dropped on a glass slide, and allowed to dry naturally. (Hospitals with cond...
Embodiment 3
[0137] Parallel experiment between detecting bladder cancer metastases with PD-ECGF gene kit and CA125 gene kit.
[0138] In order to scientifically evaluate the specificity, sensitivity and accuracy of the above genes in bladder cancer metastasis. We used the method of parallel experiments to detect the mRNA of the above genes at the same time. The detection technology used nucleic acid in situ hybridization technology, using the peripheral blood of the same patient with bladder cancer metastases to simultaneously detect the PD-ECGF gene and CA125 gene, NM-024690, ( Nucleic acid in situ hybridization, immunohistochemical staining, microscopic counting, result reporting, etc. all used the same methods, steps and reagents as in the in situ hybridization technique of Example 1 and Example 2). It was found that the expression level of PD-ECGF gene in patients with metastatic bladder cancer was higher than that of CA125 gene in patients with the same disease. The results showed t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com