Solid dispersion containing celecoxib as well as preparation method and application thereof
A technology of solid dispersion and celecoxib, which is applied in the field of solid dispersion containing celecoxib and its preparation, and can solve the problems of limited total solution, instability, and increased cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
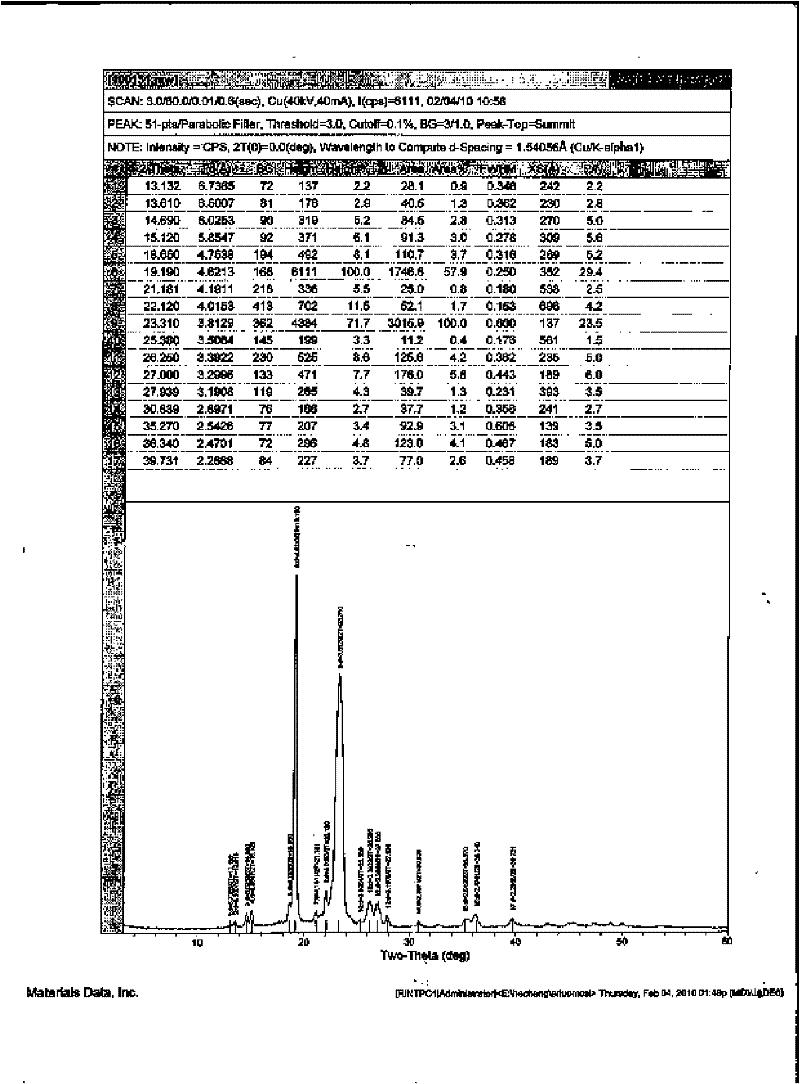
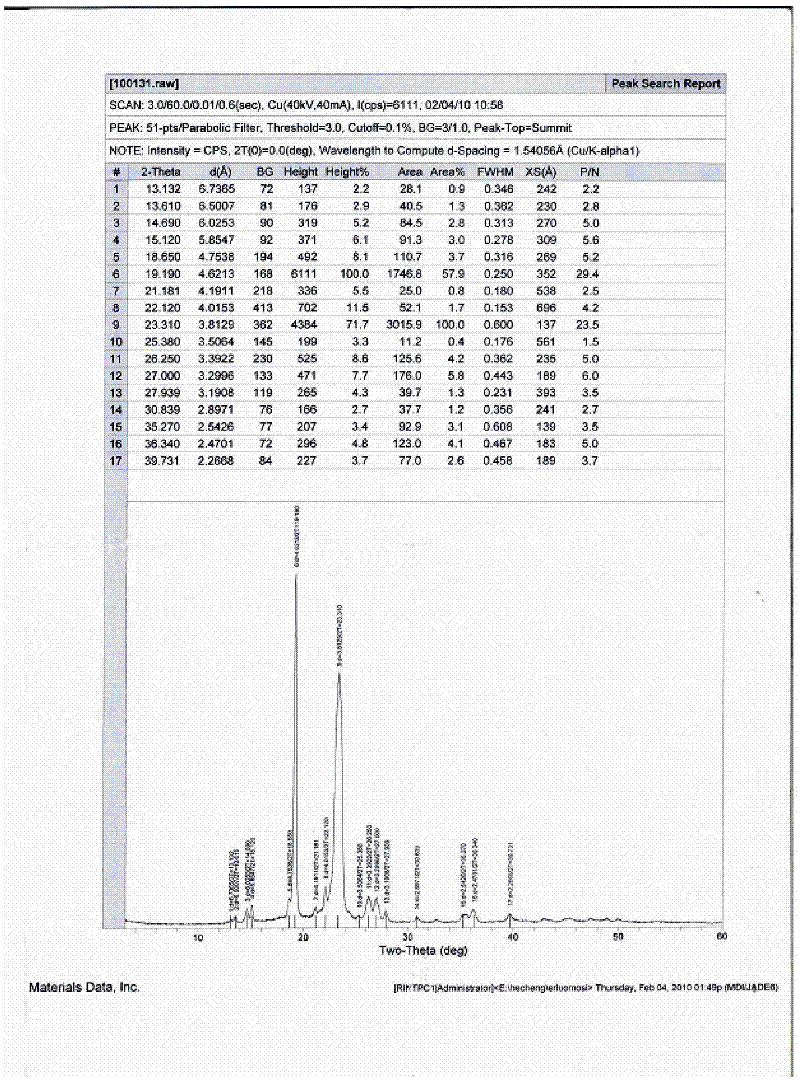
Image
Examples
Embodiment Construction
[0008] The technical problem to be solved in the present invention is to overcome the disadvantages that celecoxib crude drug is difficult to pulverize, and it is not easy to prepare solid oral preparations, and provide a solid dispersion comprising celecoxib and polyethylene glycol, which is convenient for preparing A solid oral dosage form of celecoxib.
[0009] The second problem to be solved by the present invention is to develop a method for preparing a solid dispersion comprising celecoxib and polyethylene glycol.
[0010] The third technical problem to be solved by the present invention is to develop the application of the solid dispersion containing celecoxib in the preparation of oral preparations.
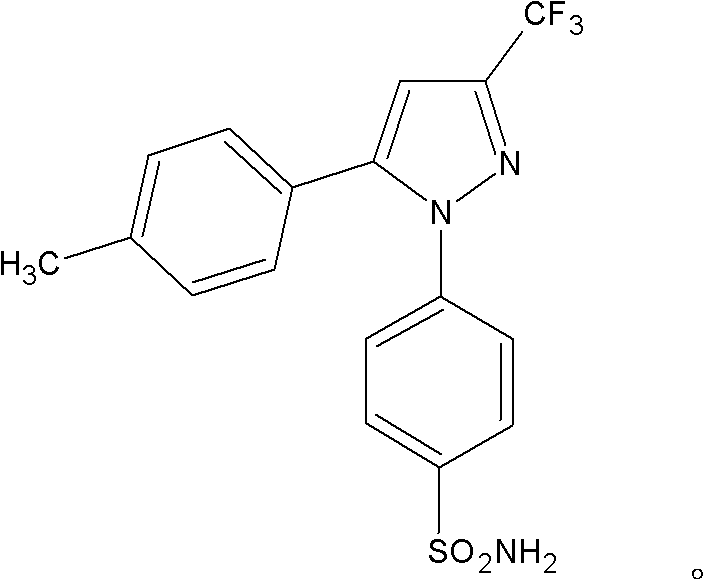
[0011] The solid dispersion containing celecoxib of the present invention is characterized in that a solid dispersion containing celecoxib and polyethylene glycol, the weight ratio of celecoxib and solid polyethylene glycol in the solid dispersion 1:0.5-10, preferably 1:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com