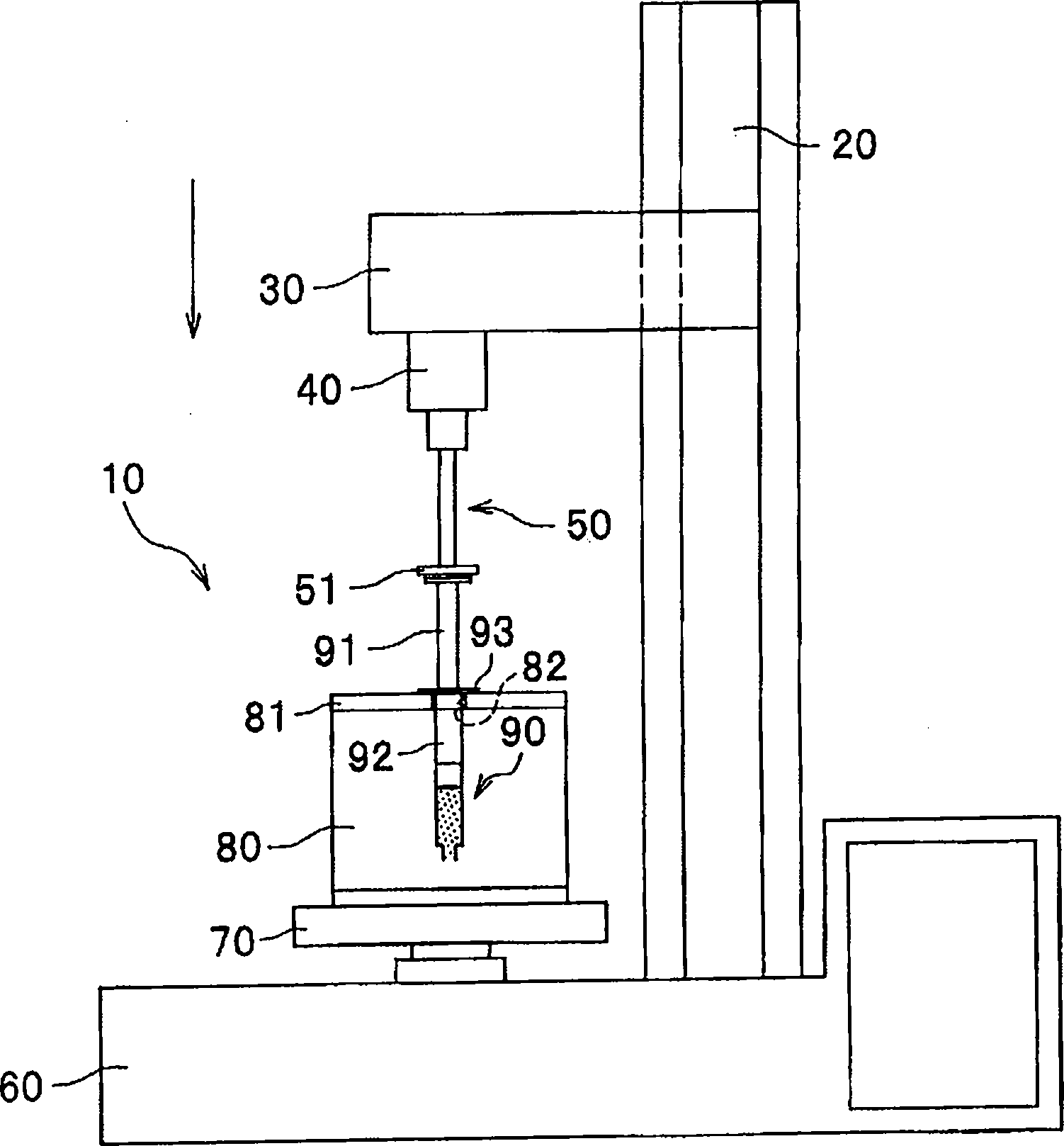
Composition for precious metal sintering, process for producing precious metal sinter and precious metal sinter
A precious metal and composition technology, applied in the field of precious metal sintering composition, can solve the problems of shape limitation, inability to use perlite or vermiculite, and lower value of precious metal products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] An 8% by weight organic binder solution consisting of 8.75% by weight of starch, 10% by weight of cellulose, and water of the remainder and 50% by weight (46% by weight of the whole) of an average particle size of 2.5 μm A mixture of Ag powder, 50% by weight (46% by weight of the whole) of Ag powder with an average particle diameter of 20 μm, and 92% by weight of silver mixed powder was used as the silver base composition.
[0137] 0.2 g of hollow glass powder (Glass Bubbles manufactured by Sumitomo 3M Co., Ltd.: bulk density of 0.075 g / cm 3 , the true density is 0.125g / cm3 , the particle size is 65μm, and the bulk volume of 0.2g in the state of being alone is 2.67cm 3 ) to make a composition for silver sintering.
[0138] Based on the volume and weight when the silver sintering composition was shaped into a cube, the density of the silver sintering composition was found to be 5.51 g / cm 3 .
[0139] The silver sintering composition is filled to an inner diameter of ...
Embodiment 2~8
[0146] Appropriately change the addition amount and size of the hollow glass powder to the conditions shown in Table 2, so that the bulk volume of the hollow glass powder accounts for the range of 5% to 160% of the whole composition. 1 is carried out in the same manner. The firing conditions were set at 600°C for 30 minutes, and the results are shown in Table 2 and Table 3.
Embodiment 9
[0163] A mixture of 8% by weight of organic binder solution consisting of 8.75% by weight of starch, 10% by weight of cellulose and the rest of water and 92% by weight of Au powder with an average particle size of 4.5 μm was used as the basic gold composition.
[0164] 5.4 g of hollow glass powder (Glass Bubbles manufactured by Sumitomo 3M Co., Ltd.: bulk density of 0.378 g / cm2) was mixed in 94.6 g of the gold basic composition 3 , the true density is 0.6g / cm 3 , particle size of 27 μm), made into a composition for gold sintering.
[0165] This gold sintering composition was molded with a silicon mold, and fired in an electric furnace at 800° C. for 30 minutes. The weight of the sintered body obtained by firing was 31.6 g, and the weight of the sintered body of the same volume without adding the hollow glass powder was 52.3 g, so the weight decreased by 40.0%. The results are also listed in Table 2.
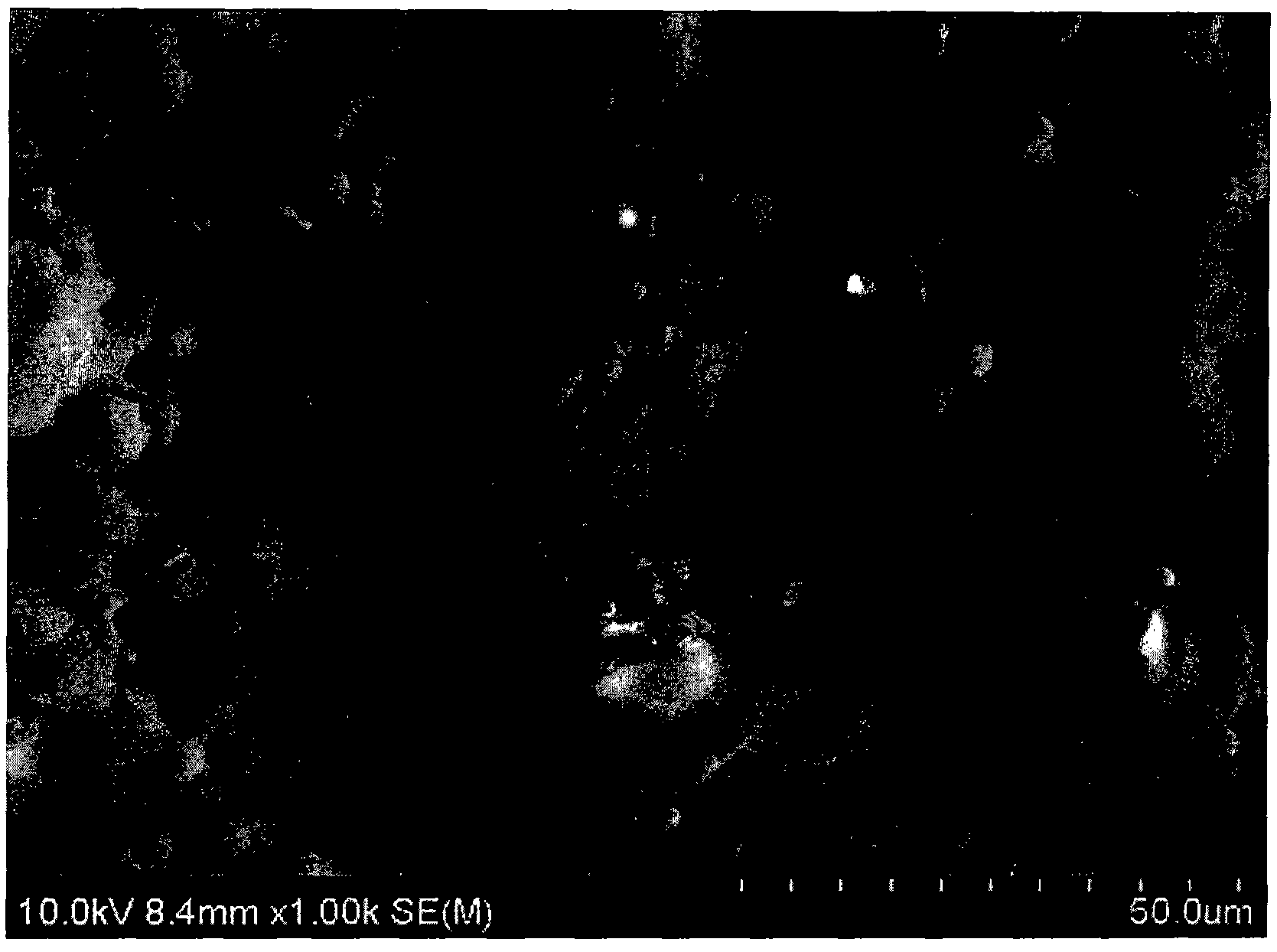
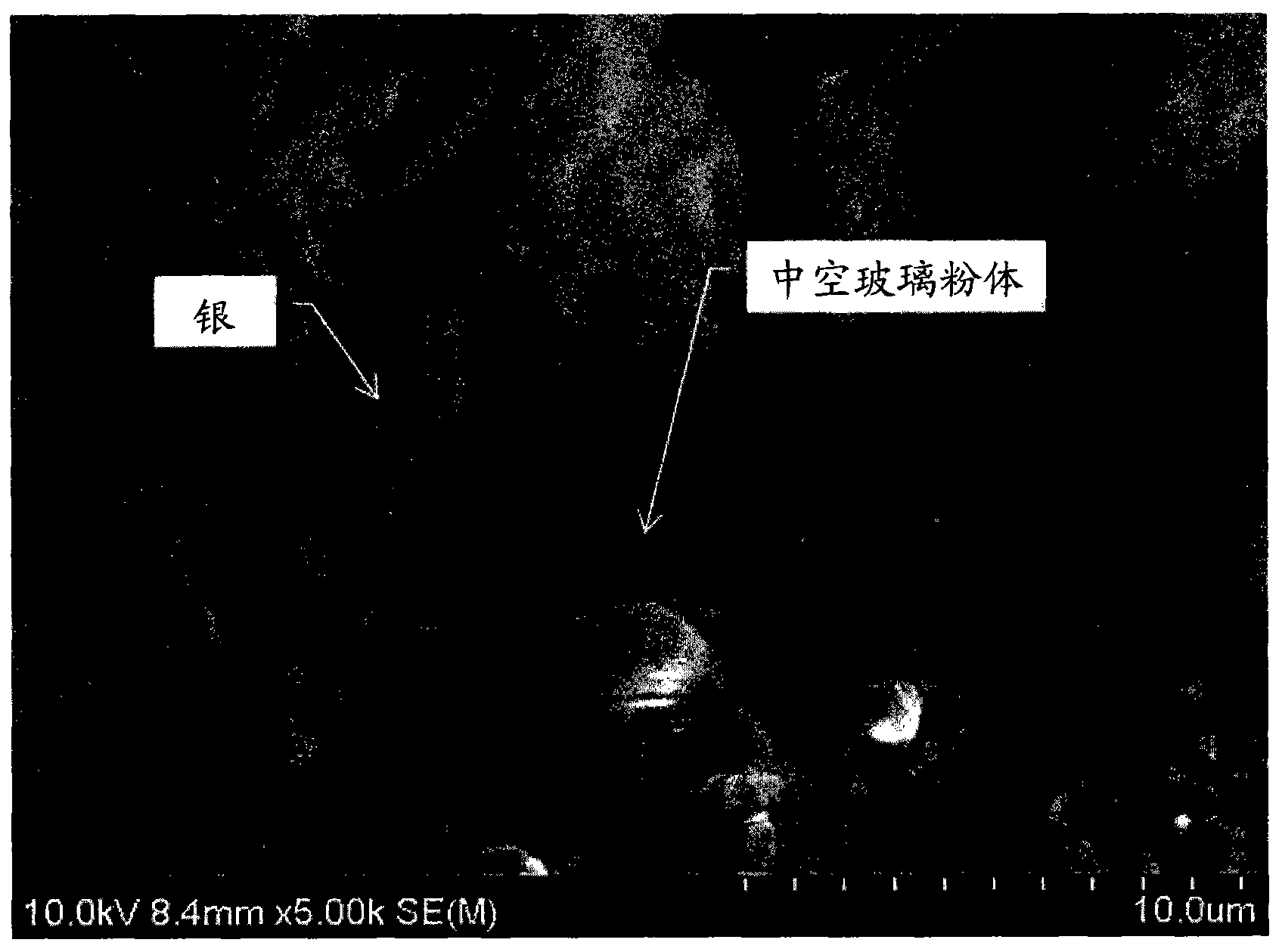
[0166] The finished samples were tumble milled. As a result, metallic lu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com