Method for decomposing scheelite by using phosphoric acid
A technology of phosphoric acid decomposition and scheelite, which is used in the extraction of rare high melting point metal tungsten and the field of phosphoric acid decomposition of scheelite. It can solve problems such as unseen industrial applications, save the cost of phosphorus removal reagents and tungsten loss, and simplify operations. , the effect of saving resources and energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
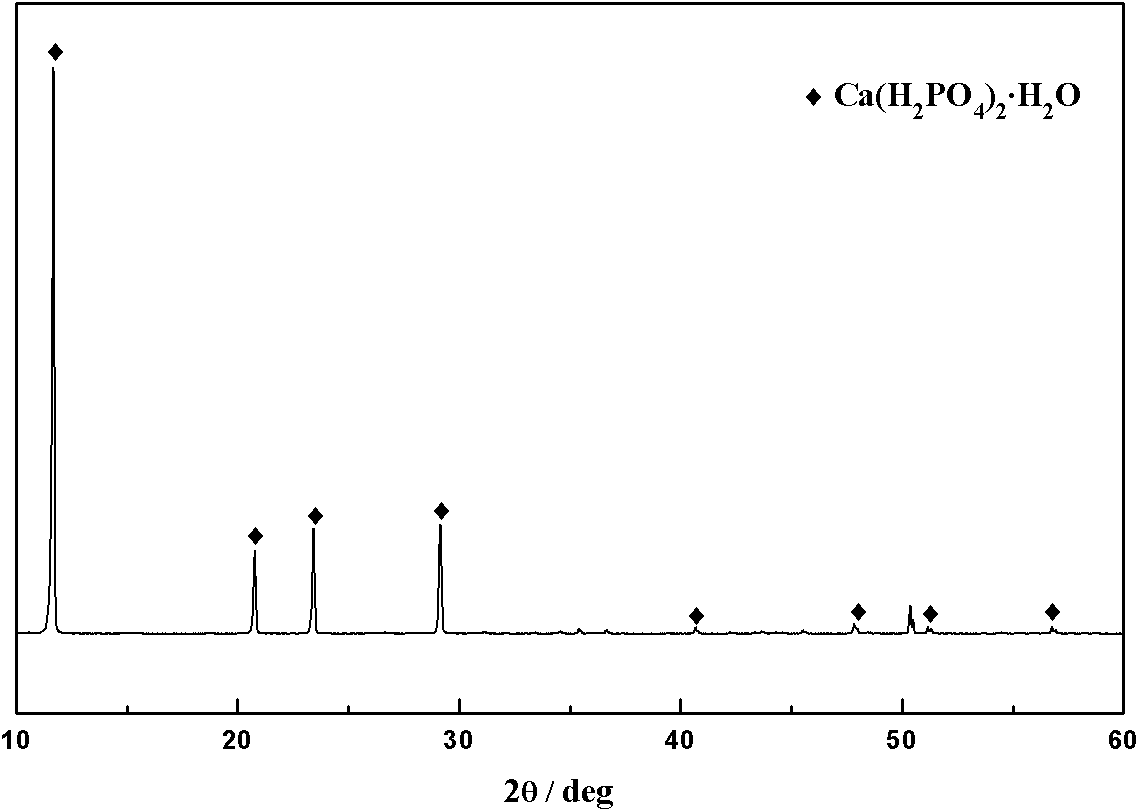
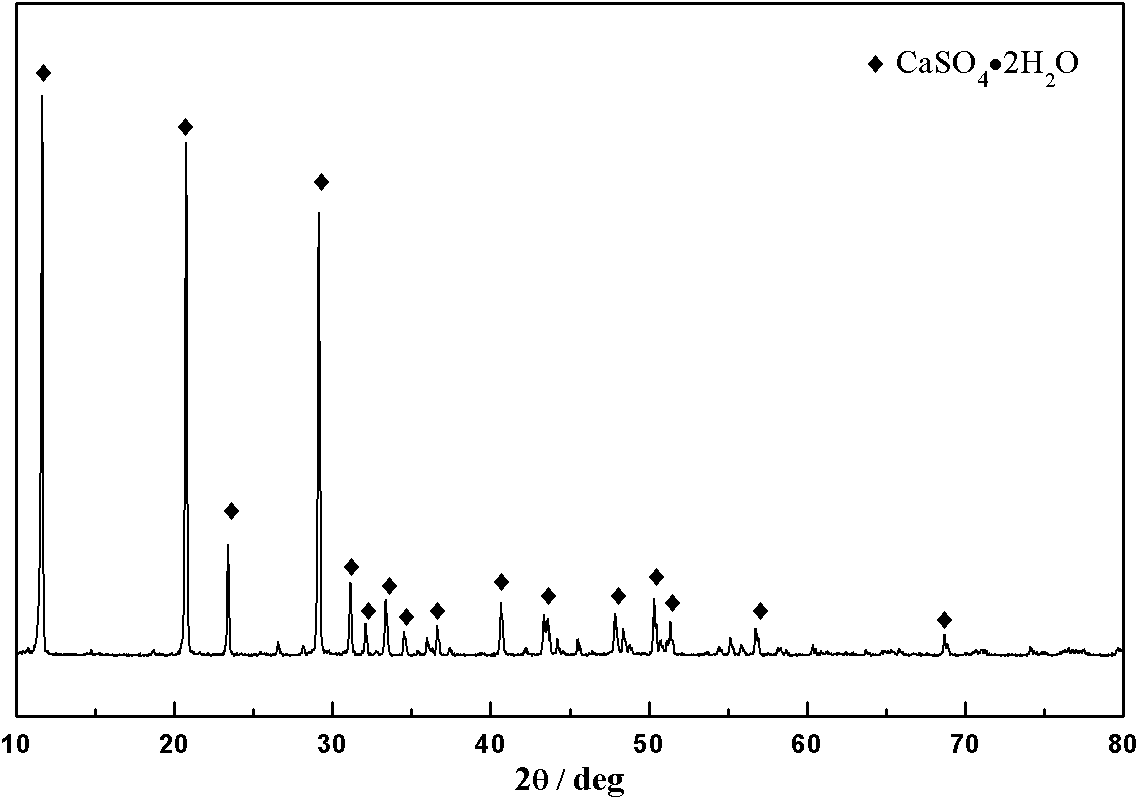
[0029] Scheelite (including WO 3 70.6%) 1kg, solid-to-liquid ratio with phosphoric acid solution 1:10g / ml, P in phosphoric acid solution 2 o 5 The content is 20%, the reaction temperature is 90°C, and the reaction time is 4h. The leaching rate of tungsten is 98.9%. The XRD pattern of the filter residue is as follows figure 1 , indicating that phosphorus is mainly in the form of Ca(H 2 PO 4 ) 2 ·H 2 The form of O is lost in the filter residue. Add 50g of phosphate rock (P 2 o 5 content is 25%), adopt sulfuric acid to decompose and reclaim phosphoric acid, the XRD pattern of solid product is as follows figure 2 , indicating that the filter residue is mainly transformed into CaSO after being treated with sulfuric acid 4 2H 2 O, P in the analyzed product 2 o 5 When the content is only 0.6%, the phosphorus enters the solution and is recovered. The filtrate is adsorbed by primary amino anion exchange resin, and the adsorption rate of tungsten is 99.3%. During the ex...
Embodiment 2
[0031] Scheelite (including WO 3 70.6%) 1kg, solid-to-liquid ratio with phosphoric acid solution 1:15g / ml, P in phosphoric acid solution 2 o 5 The content is 30%, the reaction temperature is 100°C, and the reaction time is 2h. The leaching rate of tungsten is 99.1%. After the filter residue is decomposed with sulfuric acid to recover phosphoric acid, the P in the residue 2 o 5The content was reduced to 0.7%. The filtrate is adsorbed by secondary amine-based anion exchange resin, and the adsorption rate of tungsten is 98.9%. The total loss of phosphorus in the extraction process is 4.3%, and the mother liquor returns to leaching after adding the lost phosphoric acid.
Embodiment 3
[0033] Scheelite (including WO 3 70.6%) 1kg, solid-to-liquid ratio with phosphoric acid solution 1:20g / ml, P in phosphoric acid solution 2 o 5 The content is 10%, the reaction temperature is 60°C, and the reaction time is 10h. The leaching rate of tungsten is 98.6%. After the filter residue is decomposed with sulfuric acid to recover phosphoric acid, the P in the residue 2 o 5 The content was reduced to 0.9%. The filtrate is adsorbed by a tertiary amine-based anion exchange resin, and the adsorption rate of tungsten is 98.4%. During the extraction process, phosphorus is lost in the form of phosphotungstic heteropolyacid and the total amount of entrainment loss is 4.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com