In vitro detection method and application of antibody
An in vitro detection and antibody technology, applied in measuring devices, color/spectral characteristic measurement, instruments, etc., can solve the problems that cannot meet the needs of large-scale in vitro detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1Protein A is coated on microtiter plate
[0068] Select an untreated polystyrene microtiter plate, add 0.9μg / ml Protein A dissolved in carbonate buffer, pH9.2-9.8, 100μl / well, overnight at 4°C. The coating solution was removed, and 200 μl of 1% (w / v) BSA solution (PBS, pH 7.4) was added to each well for blocking, overnight at 4°C or 1-2 hours at 37°C.
Embodiment 2
[0069] Embodiment 2 Serum sample detection method
[0070] Wash the microtiter plate 1-2 times with PBST (PBS buffer containing 0.05% Tween-20), and pat dry each time.
[0071] After adding 50 μl of PBS to each well, add 50 μl of serum to be tested into each well, mix well and incubate at 37° C. for 60 minutes.
[0072] After the reaction solution was removed and patted dry, the plate was washed 4 times with PBST, each time patted dry.
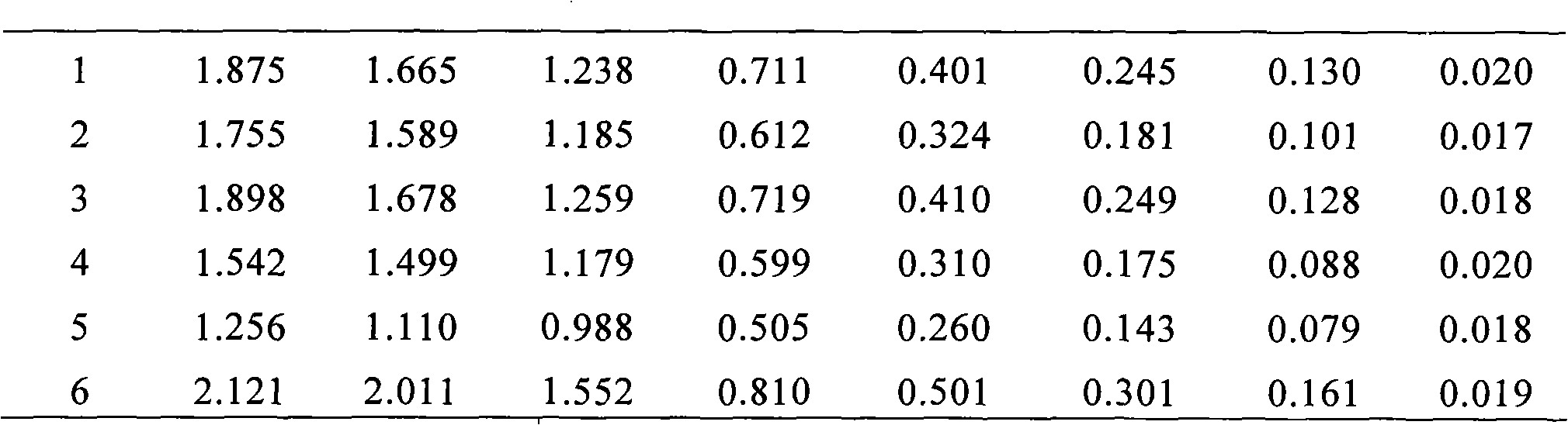
[0073] Add 50 μl recombinant HBeAg (SEQ ID NO: 2, provided by Shanghai Rongsheng Bio-Pharmaceutical Co., Ltd.) (diluted 1:6000 with PBS) and 50 μl HBeAg antibody labeled with HRP (diluted 1:4000 with PBS) to each well.
[0074] Add 50 μl each of substrate A and B solution to each well, mix well, incubate at 37°C for 15 minutes, then add stop solution, and measure with a 450nm microplate reader.
[0075] Substrate A solution preparation:
[0076] Weigh 17.9g of citric acid and Na 2 HPO 4 12 2 O 4.67g was dissolved in 400ml deionized water...
Embodiment 3
[0082] Embodiment 3HBeAb detects parallel control
[0083] The currently used HBeAb competition ELISA detection kit (Shanghai Rongsheng Biopharmaceutical Co., Ltd.) was used for comparison.
[0084] A total of 350 specimens were used in this experiment, among which,
[0085] Da San Yang (positive for HBsAg, HBeAg and HBcAb): 110 cases;
[0086] Small Sanyang (positive for HBsAg, HBeAb and HBcAb): 100 cases;
[0087] Healthy human serum (from blood bank): 140 cases,
[0088] The test results are shown in Table 1.
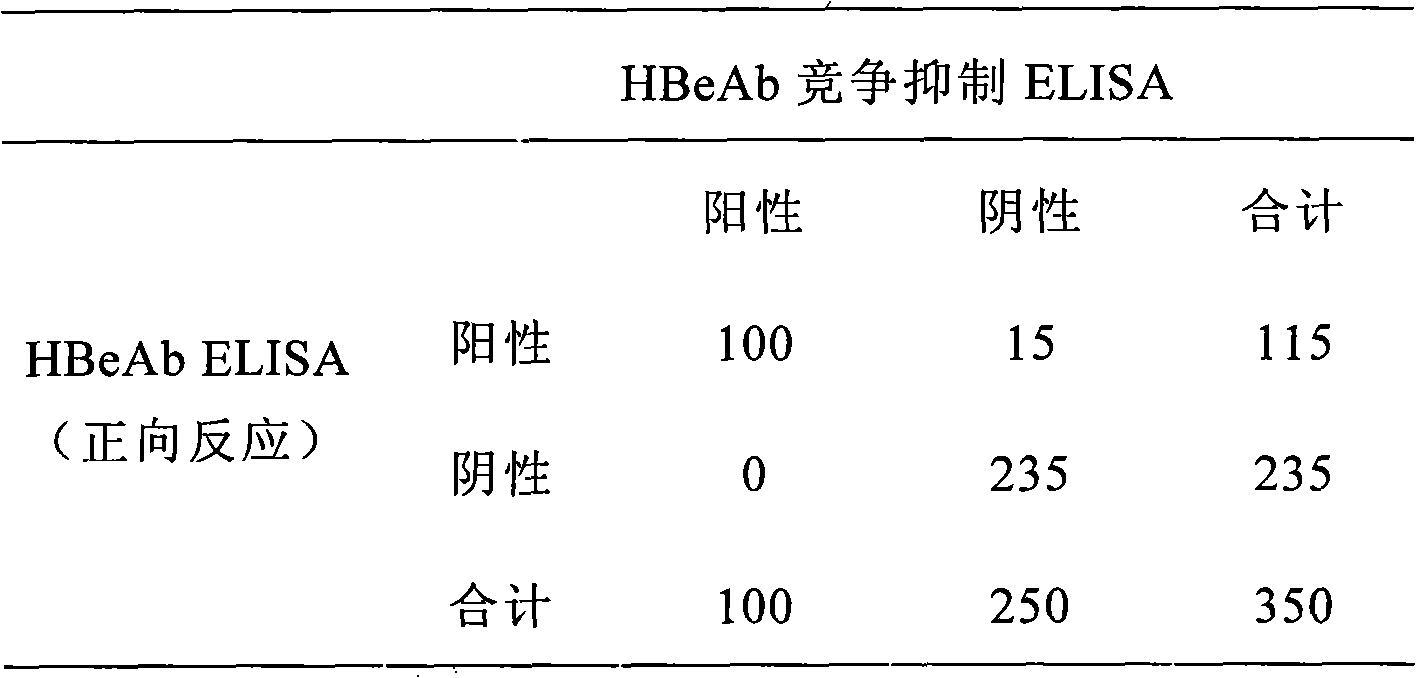
[0089] Table 1 HBeAb detection parallel control
[0090]
[0091] It can be obtained from Table 1:
[0092] Positive consistency rate: 100 / 100=100%;
[0093] Negative consistency rate: 235 / 250=94%;
[0094] The overall agreement rate: 335 / 350=95.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com