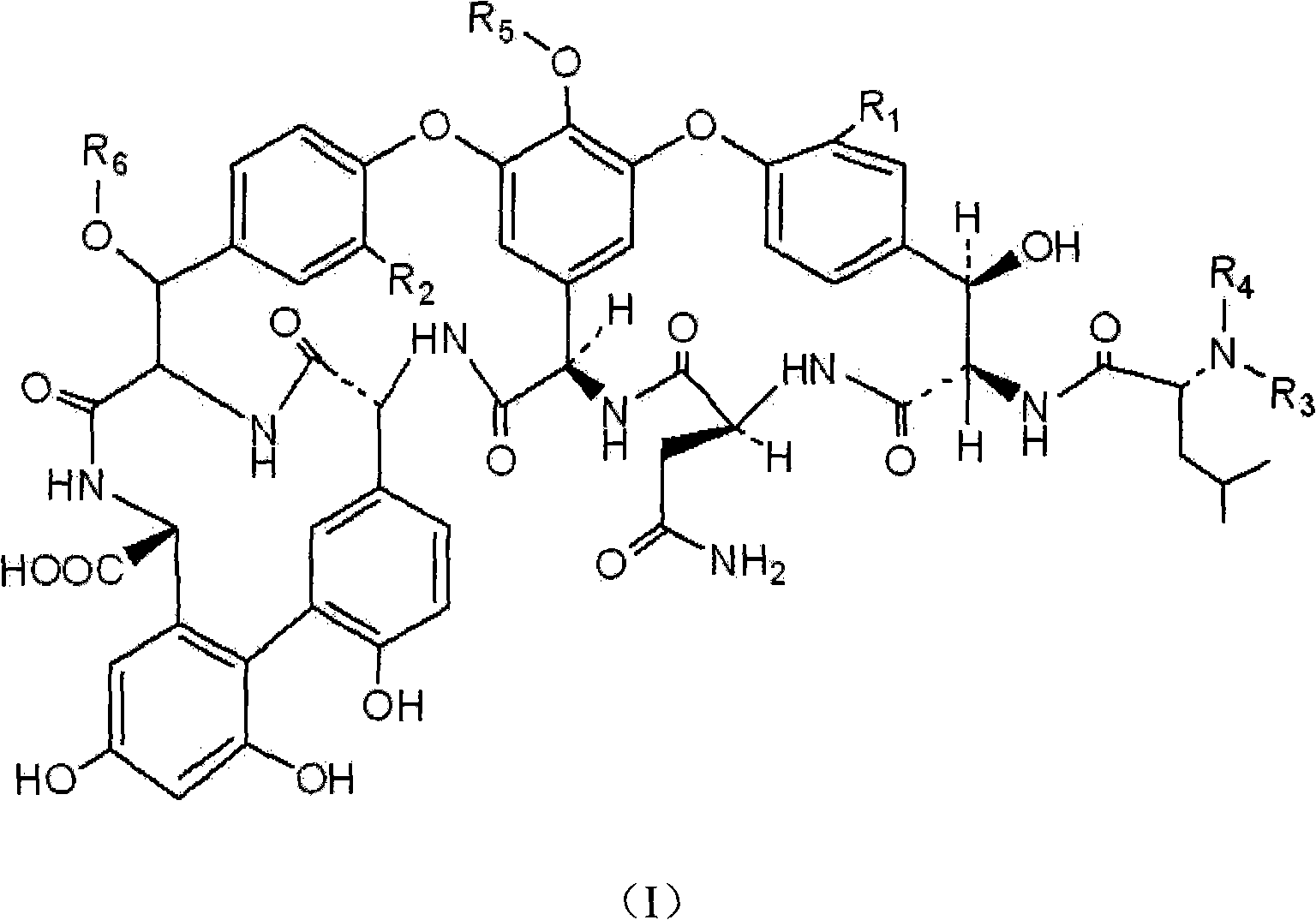
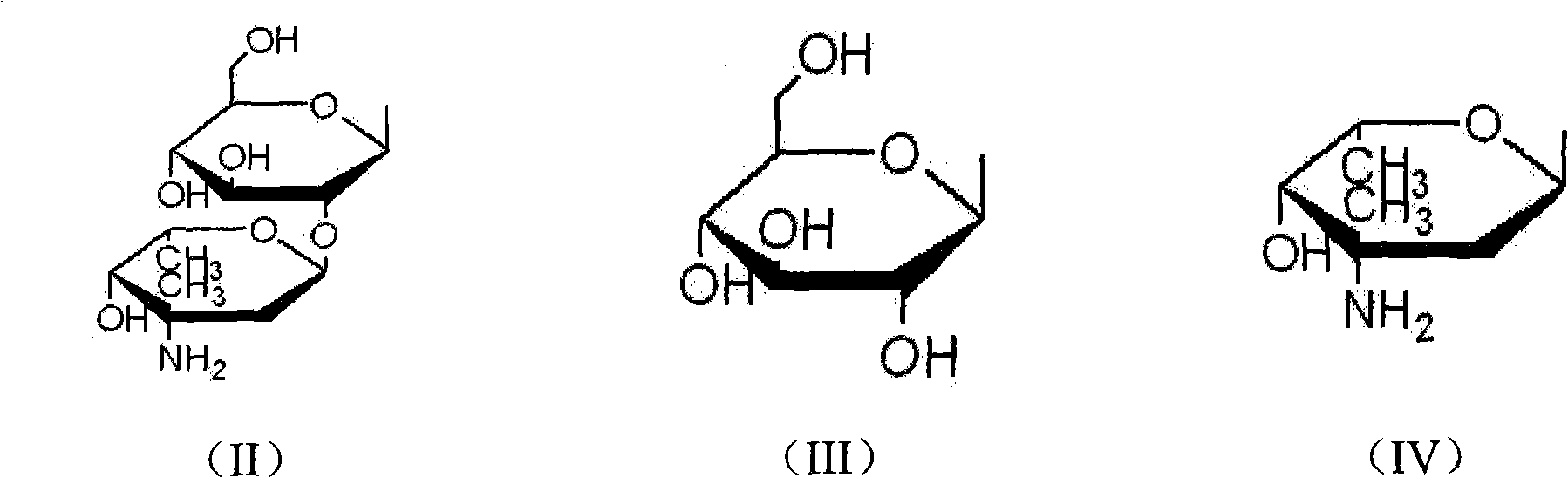
Method for purifying glycopeptide compound
A purification method and compound technology, which is applied in the field of glycopeptide compounds, can solve the problems of slow desorption speed, large difference in swelling coefficient, tailing, etc., and achieve the effects of reducing yield loss, shortening purification cycle, and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 , Compound 3 preparation and crude purity
[0028] 1.1. Preparation
[0029] Referring to the fermentation method in the Chinese Invention Patent Application No. 200910053906.9, the fermentation broth of compound 3 was prepared, and the titer was 0.3 mg / ml after testing.
[0030] 1.2, crude and pure
[0031] Take 20L of the fermentation broth prepared in step 1.1, adjust the pH to 9.0 and filter, load the obtained clarified filtrate into 500ml of macroporous adsorption resin XAD-1600 (Rohm & Haas), and then wash the resin with 8% ethanol aqueous solution.
[0032] Then eluted with 2L 0.05% HCl aqueous solution, collected the eluate, concentrated to 500ml with a nanofiltration membrane with a 300Da pore size, and then added 100g of gel-type cation exchange resin 001×4 for static decolorization for 6 hours.
[0033] The resin was filtered off, and the decolorized solution was lyophilized to obtain 4.3 g of a crude product of compound 3.
[0034] After testin...
Embodiment 2-10
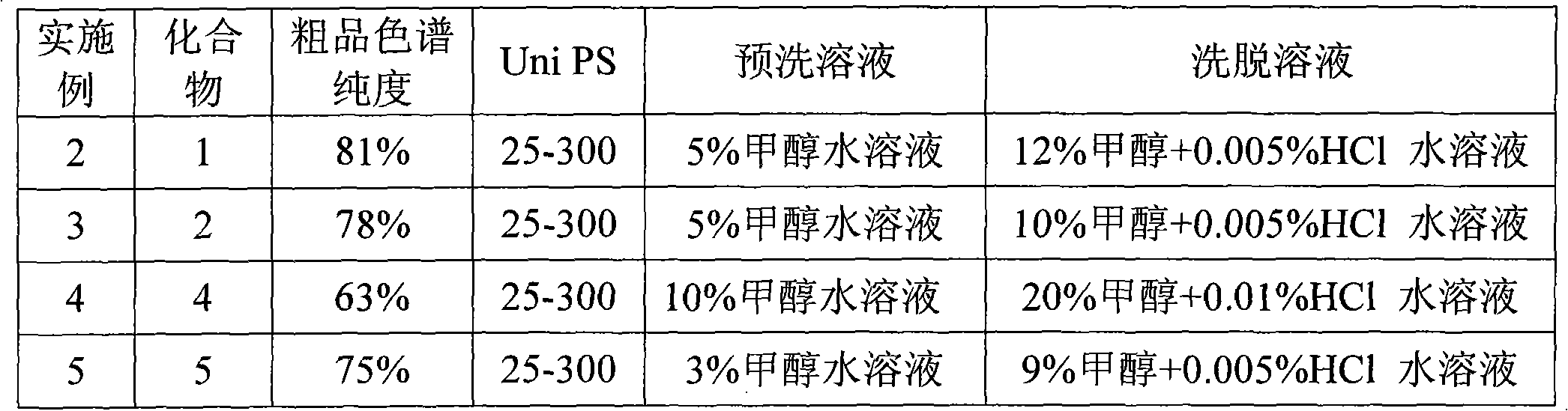
[0035] Examples 2-10 , Compounds 1, 2, 4-8 are pure
[0036] Take 0.20 g of each of the crude products of compounds 1, 2, 4-8 shown in Table 2, dissolve them in 10 ml of pure water, and load the samples onto a 10 mm × 20 cm glass chromatography column equipped with 10 ml of reversed-phase polymer filler Uni PS. , the sample flow rate was 10 ml / h. After sample loading, pre-wash with 10 ml methanol aqueous solution for 1 h, and then elute with methanol aqueous solution with or without HCl at a flow rate of 20 ml / h. After elution of 6ml, the eluate was collected, and a total of 15ml of eluate was collected. The eluate was concentrated and dried to obtain the purified products of compounds 1, 2, 4-8, respectively. The chromatographic purity of the crude product and the middle of the purification step The used pre-wash solution and elution solution are shown in Table 2, respectively. The purified products of the above compounds obtained were detected and the yields of the above ...
Embodiment 11-19
[0043] Examples 11-19 , Compound 3 is pure
[0044] Take 6 parts of the crude product of compound 3 obtained in Example 1, each 0.20 g, dissolve in 10 ml of pure water, respectively, and then load the samples into a 10 mm × 20 cm glass chromatography column equipped with 10 ml of reversed-phase polymer filler Uni PS. , after the sample loading, pre-wash with 10ml methanol aqueous solution for 1h, and then elute with methanol aqueous solution with or without HCl. After eluting 6ml, start to collect the eluent, and collect a total of 15ml of eluent, and then eluate the eluate Concentrate and dry to obtain the purified product of compound 3, the purification step parameters are shown in Table 4, and the obtained purified product of compound 3 is detected, and the detection results are shown in Table 5.
[0045] Table 4. Refinement parameters
[0046]
[0047] Table 5, the detection result of purified product
[0048] Example
Yield
color
purity
yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com