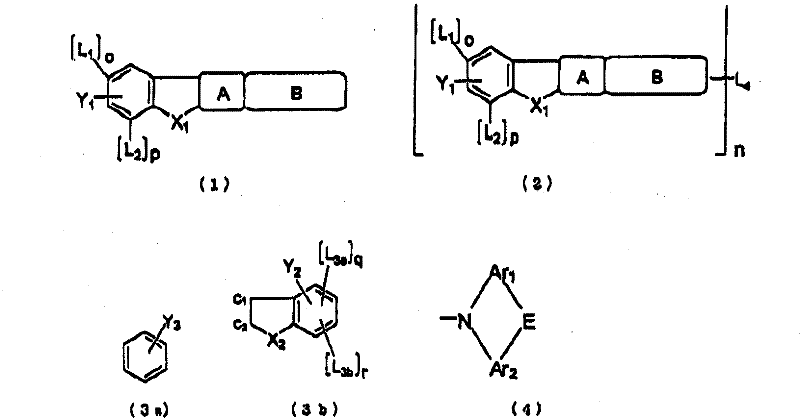
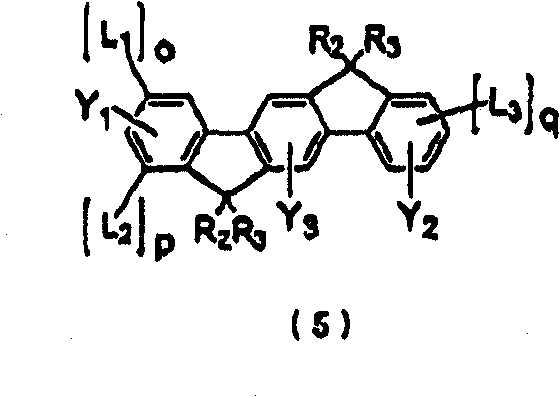
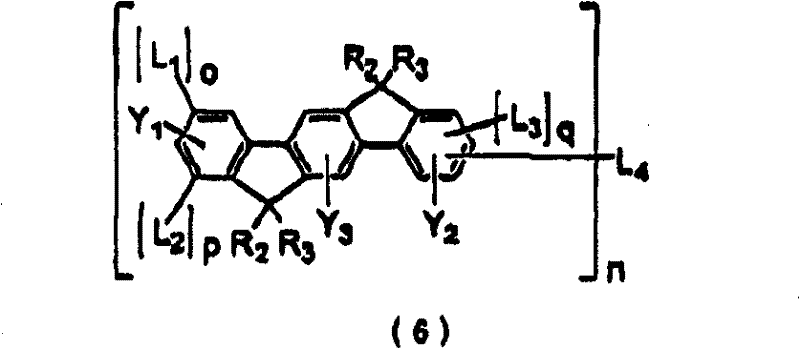
Polycyclic compound and organic electroluminescent device using the same
A polycyclic compound and organic technology, applied in the field of organic electroluminescent components and polycyclic compounds, can solve the problems of poor efficiency and insufficient practical use, etc., and achieve the effect of long life and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0657] (Manufacturing of organic EL elements)
[0658] A 25 mm x 75 mm x 1.1 mm glass substrate with an ITO transparent electrode (manufactured by Geomatec) was subjected to ultrasonic cleaning in isopropanol for 5 minutes, followed by UV (Ultraviolet) ozone cleaning for 30 minutes.
[0659] The glass substrate with the transparent electrode after cleaning in this way is mounted on the substrate holder of the vacuum evaporation device, first on the surface of the side of the glass substrate where the transparent electrode line is formed, the compound 1-A is evaporated with a thickness of 30nm, This was made to cover the transparent electrode to obtain a hole transport layer.
[0660] On this hole transport layer, compound 1-1 as a host for phosphorescence and Ir(Ph-ppy)3 as a dopant for phosphorescence were co-deposited to a thickness of 30 nm to obtain a phosphorescence emitting layer. The concentration of Ir(Ph-ppy)3 was 5% by mass.
[0661] Next, compound 1-B with a thick...
Embodiment 1-2~1-19
[0667] An organic EL device was fabricated and evaluated in the same manner as in Example 1-1, except that the host material described in Table 1 was used instead of the host compound 1-1 in Example 1-1. Table 1 shows the evaluation results of luminous performance.
Embodiment 2-1
[0885] (Manufacturing of organic EL elements)
[0886] A 25 mm x 75 mm x 1.1 mm ITO transparent electrode-attached glass substrate (manufactured by Geomatec) was subjected to ultrasonic cleaning in isopropanol for 5 minutes, followed by UV (Ultraviolet) ozone cleaning for 30 minutes.
[0887] The glass substrate with the transparent electrode after cleaning in this way was installed on the substrate holder of the vacuum evaporation device, first on the surface of the side of the glass substrate where the transparent electrode line was formed, the following compound 2- A, making it cover the transparent electrode to obtain a hole transport layer.
[0888] Compound 2-1 as a host material for phosphorescence and Ir(Ph-ppy)3 as a dopant for phosphorescence were co-deposited to a thickness of 30 nm on the hole transport layer to obtain a phosphorescent emitting layer. The concentration of Ir(Ph-ppy)3 was 5% by mass.
[0889] Next, compound 2-B with a thickness of 10 nm, compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com