CaF2-doped LiBH4 reversible hydrogen storage material with high hydrogen storage quantity and preparation method thereof
A hydrogen storage material and hydrogen storage technology are applied in chemical instruments and methods, hydrogen, borane/diborane hydride, etc., to achieve the effect of improving the kinetic performance of hydrogen absorption and desorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: CaF 2 Doped LiBH 4 preparation of
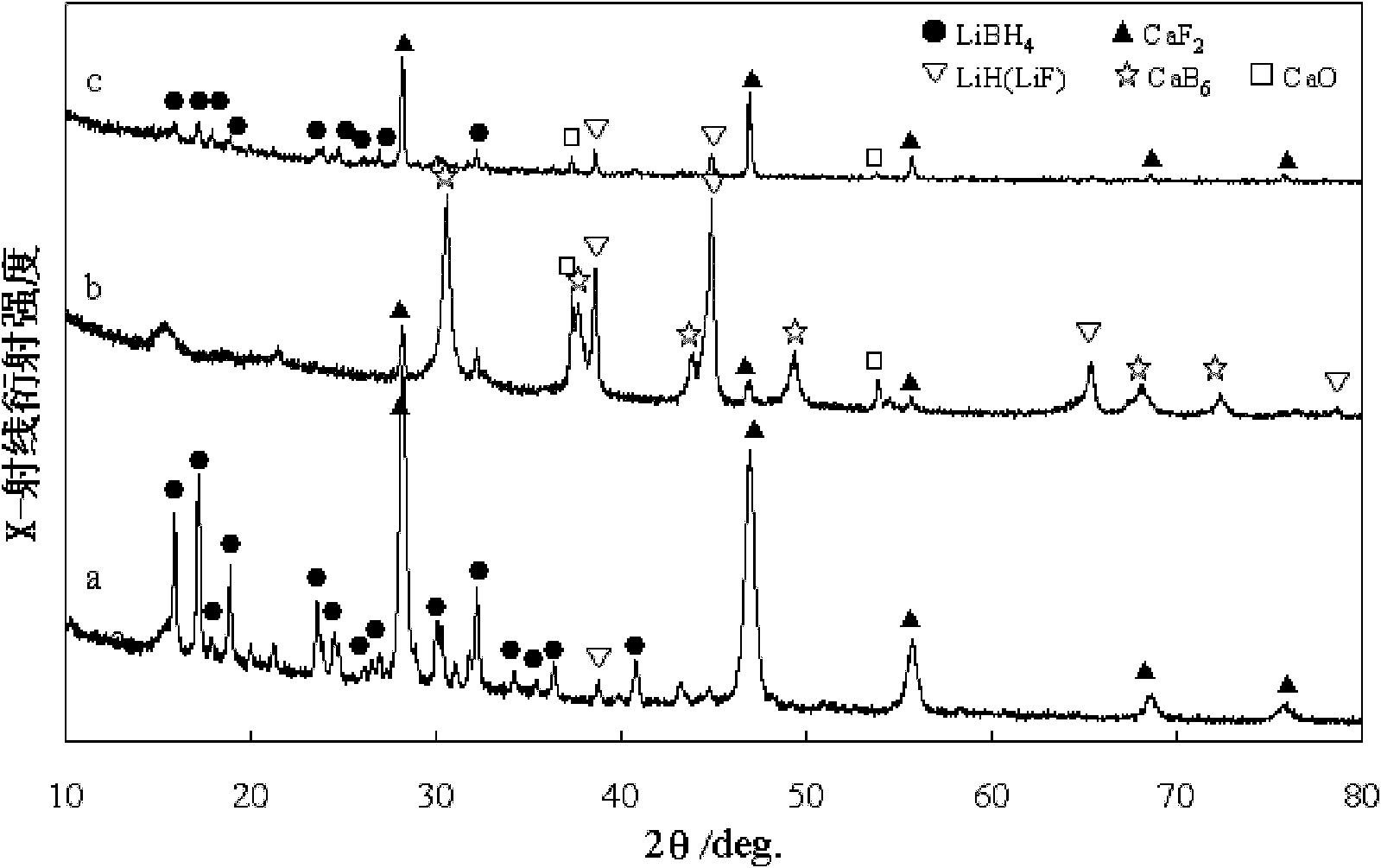
[0036] CaF 2 Powder and LiBH 4 The powder is mixed at a molar ratio of 1:6, ball milled at 1000rpm for 10 hours, filled into a stainless steel reactor, vacuumed to 1 Pascal at 450°C, maintained for 1 hour, and then filled with hydrogen at 90 atmospheres (purity 99.999%), CaF can be produced by maintaining hydrogen pressure for 10 hours 2 Doped LiBH 4 .
Embodiment 2
[0037] Example 2: CaF 2 Doped LiBH 4 Hydrogen release performance
[0038] CaF 2 Powder and LiBH 4 The powder was mixed at a molar ratio of 1:6, ball milled at 800rpm for 16 hours, filled into a stainless steel reactor, vacuumed at 450°C to 1 Pascal, maintained for 5 hours, and then filled with hydrogen at 90 atmospheres (purity 99.999%), CaF can be produced by maintaining hydrogen pressure for 10 hours 2 Doped LiBH 4 .
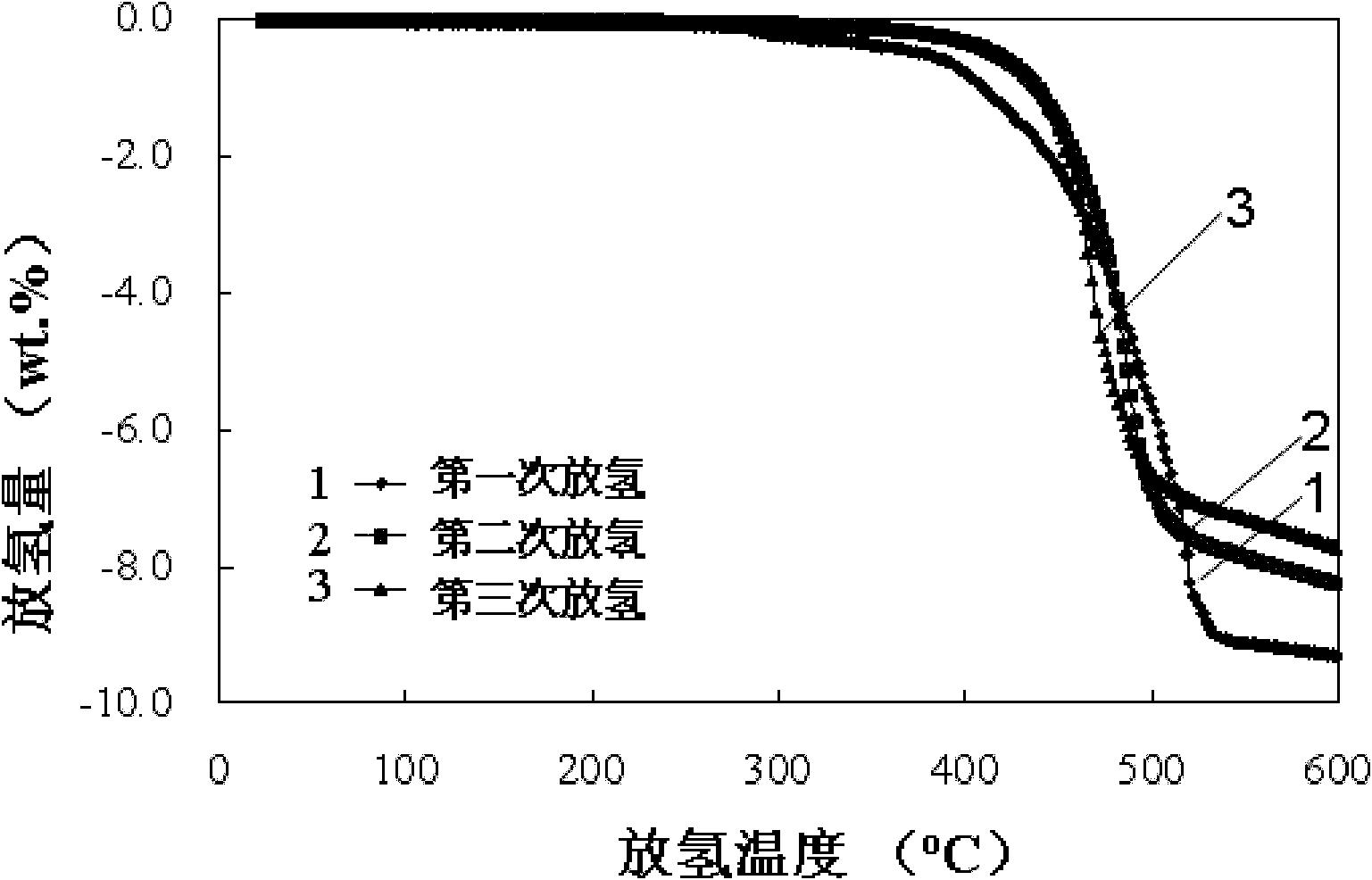
[0039] Reduce the pressure to 1atm, with 10 o Heating at a heating rate of C / min, measuring the amount of hydrogen released by a flow meter, after the completion of the hydrogen release, then filling with 90 atmospheres of hydrogen (purity 99.999%), repeating 3 times. figure 2 is the kinetic performance of the 1st, 2nd, and 3rd hydrogen desorption, it can be seen that CaF 2 Doped LiBH 4 The hydrogen release is completely reversible.
Embodiment 3
[0040] Example 3: CaF 2 Doped LiBH 4 Hydrogen absorption performance
[0041] CaF 2 Powder and LiBH 4 The powder was mixed at a molar ratio of 1:6, ball milled at 500rpm for 12 hours, filled into a stainless steel reactor, vacuumed at 450°C to 0.5 Pascal, maintained for 3 hours, and then filled with hydrogen at 90 atmospheres (purity 99.999%), CaF can be produced by maintaining hydrogen pressure for 15 hours 2 Doped LiBH 4 .
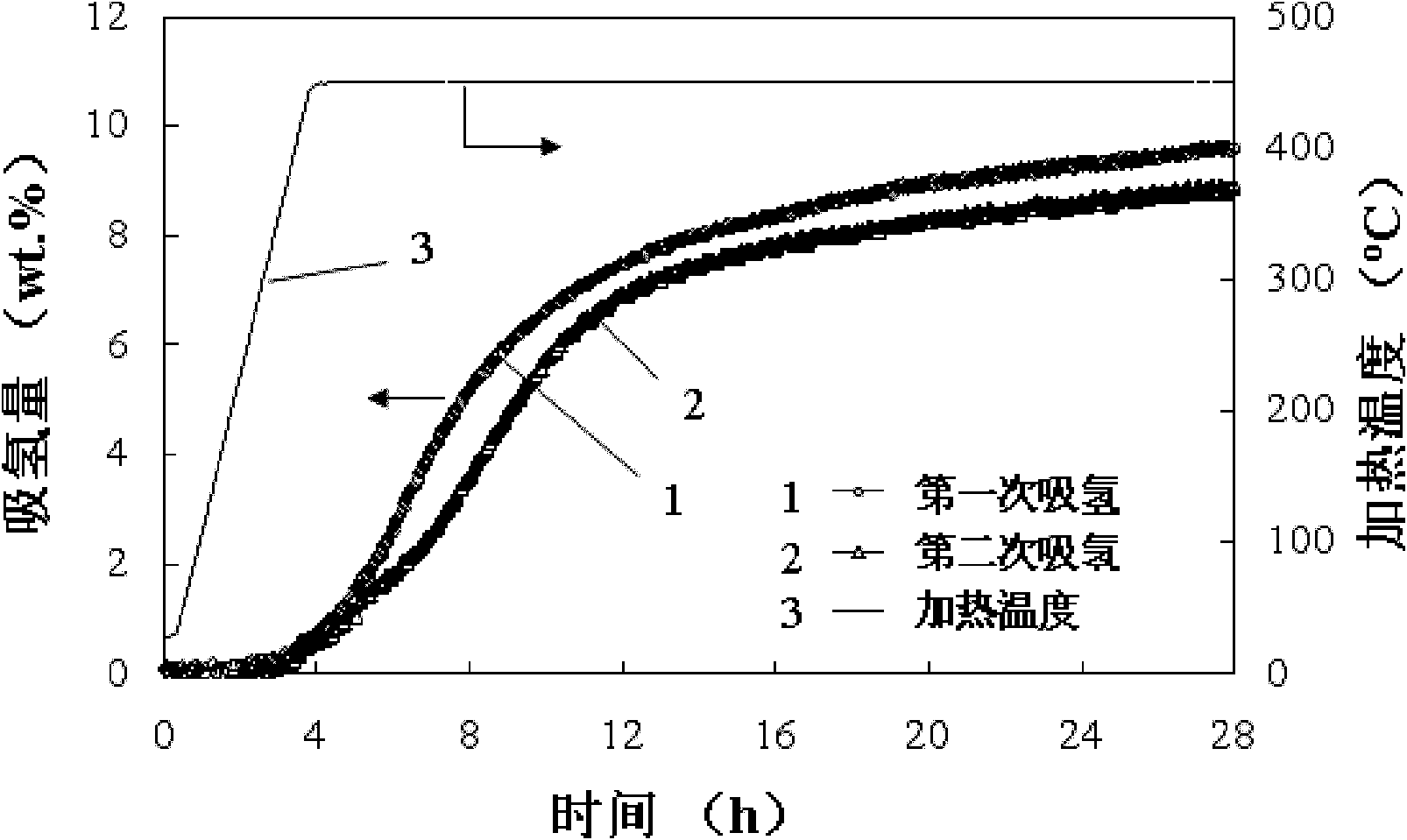
[0042] Decrease the pressure to 1atm and dehydrogenate. After the dehydrogenation is completed, fill in hydrogen at 90 atmospheres (purity 99.999%), and record the CaF 2 Doped LiBH 4 The pressure drop when absorbing hydrogen is converted into the amount of hydrogen absorbed. Repeat 2 times, image 3 is the kinetic performance of the first and second hydrogen absorption, it can be seen that CaF 2 Doped LiBH 4 The hydrogen uptake is completely reversible.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com