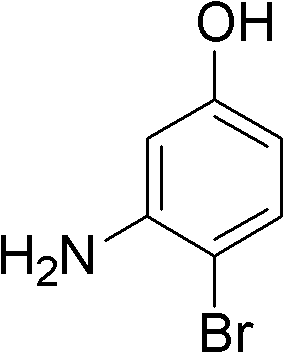
Method for synthesizing 3-amino-4-bromophenol
A synthesis method and aminophenol technology are applied in the preparation of amino hydroxy compounds, chemical instruments and methods, preparation of organic compounds, etc., which can solve the problems of complex post-processing steps, serious pollution and high cost, and achieve complex post-processing and product quality. The effect of high yield and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
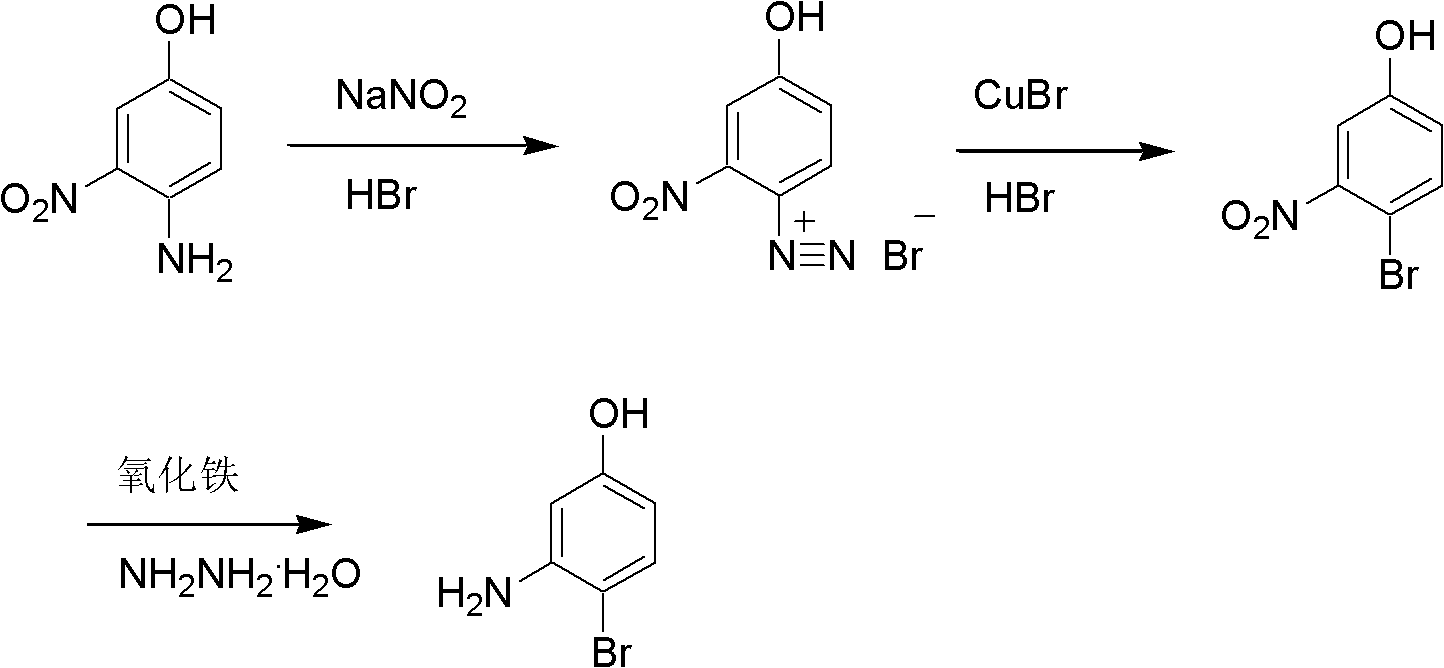
[0017] (1) Diazotization reaction: Take 12g of 3-amino-4-nitrophenol, dissolve in 64g of 40wt% hydrobromic acid aqueous solution, and cool down to 0-10°C. At this temperature, 36 g of a 20 wt % sodium nitrite aqueous solution was added dropwise. Insulation reaction 1h. The obtained yellow liquid is an aqueous solution of 3-nitrophenol-4-diazonium salt, which is directly used in the next reaction.
[0018] (2) Bromination reaction: Take 1.2 g of cuprous bromide and dissolve it in 27 g of 40 wt % hydrobromic acid aqueous solution. After the temperature was raised to 40-45° C., the reaction solution obtained in step (1) was started to be added dropwise. After the dropwise addition was completed, the reaction was incubated for 1 h. The temperature was lowered to 20-25°C, and a solid was precipitated. The product was filtered off and the solid was washed with a little water and dried. 13.1 g of 3-nitro-4-bromophenol are obtained. Content 95%.
[0019] (3) Reduction reaction:...
Embodiment 2
[0022] (1) Diazotization reaction: Take 12g of 3-amino-4-nitrophenol, dissolve in 64g of 40wt% hydrobromic acid aqueous solution, and cool down to 0-10°C. At this temperature, 36 g of a 20 wt % sodium nitrite aqueous solution was added dropwise. Insulation reaction 1h. The obtained yellow liquid is an aqueous solution of 3-nitrophenol-4-diazonium salt, which is directly used in the next reaction.
[0023] (2) Bromination reaction: Take 1.2 g of cuprous bromide and dissolve it in 27 g of 40 wt % hydrobromic acid aqueous solution. After the temperature was raised to 40-45° C., the reaction solution obtained in step (1) was started to be added dropwise. After the dropwise addition was completed, the reaction was incubated for 1 h. The temperature was lowered to 20-25°C, and a solid was precipitated. The product was filtered off and the solid was washed with a little water and dried. 13.1 g of 3-nitro-4-bromophenol are obtained. Content 95wt%.
[0024] (3) Reduction reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com