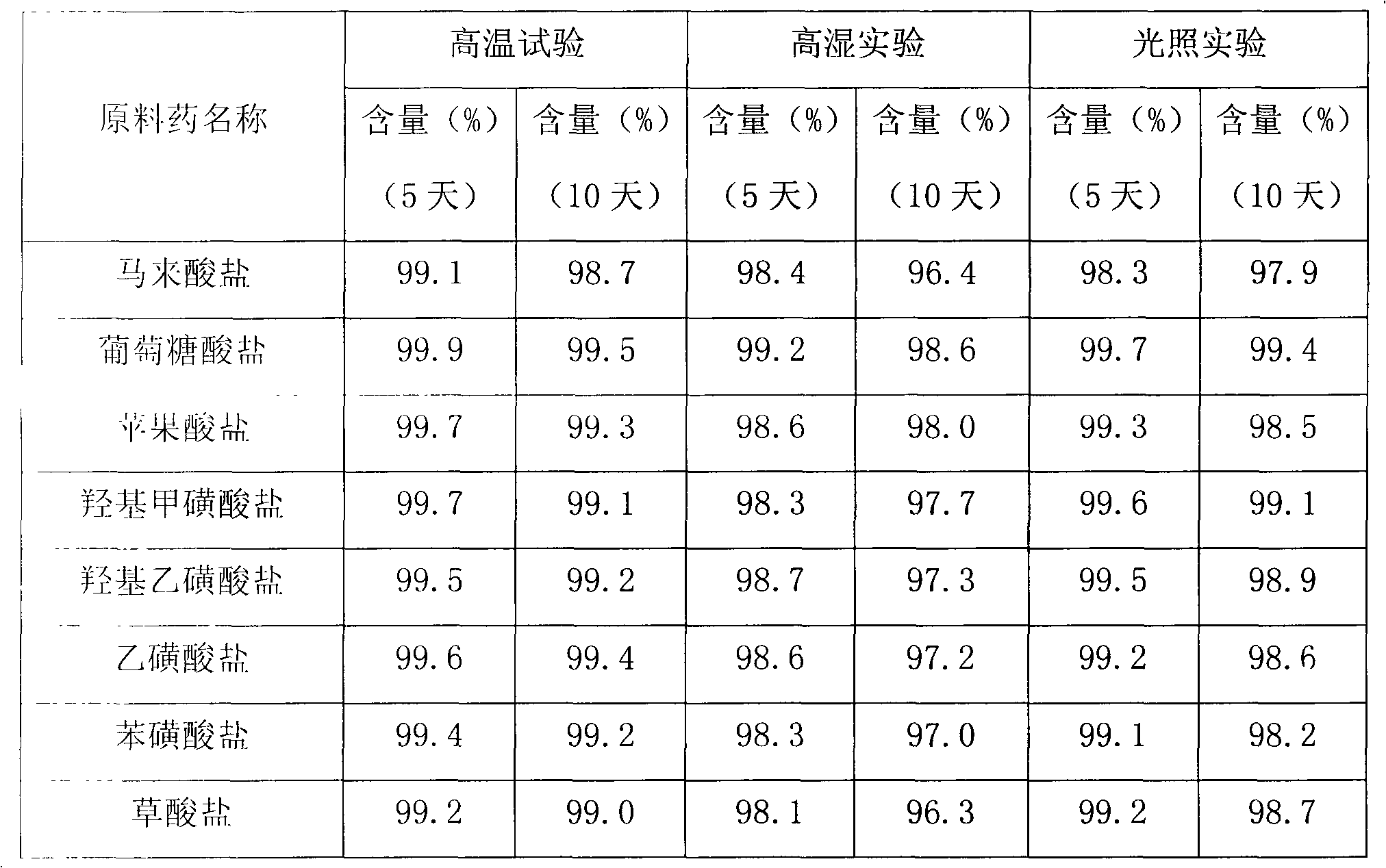
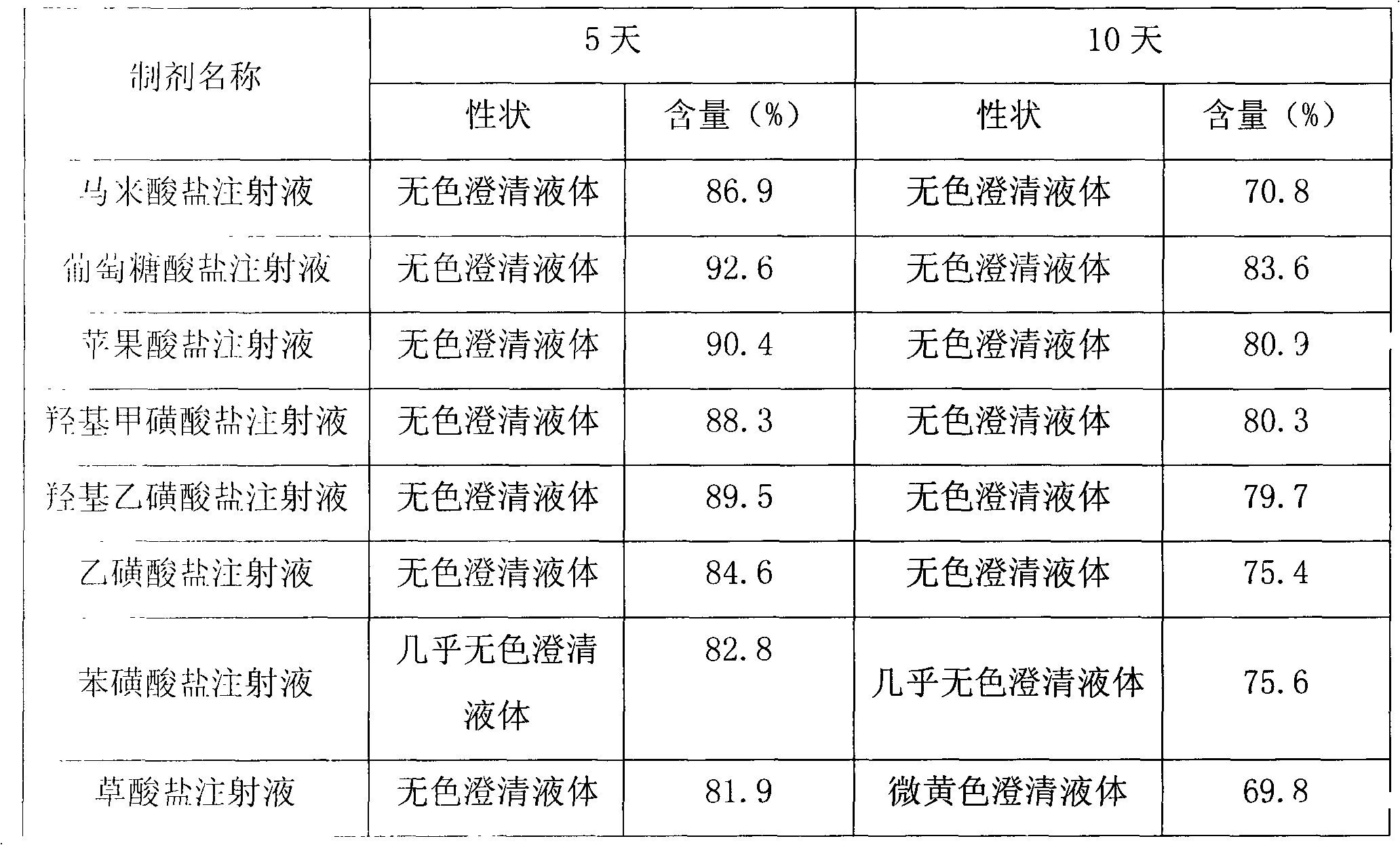
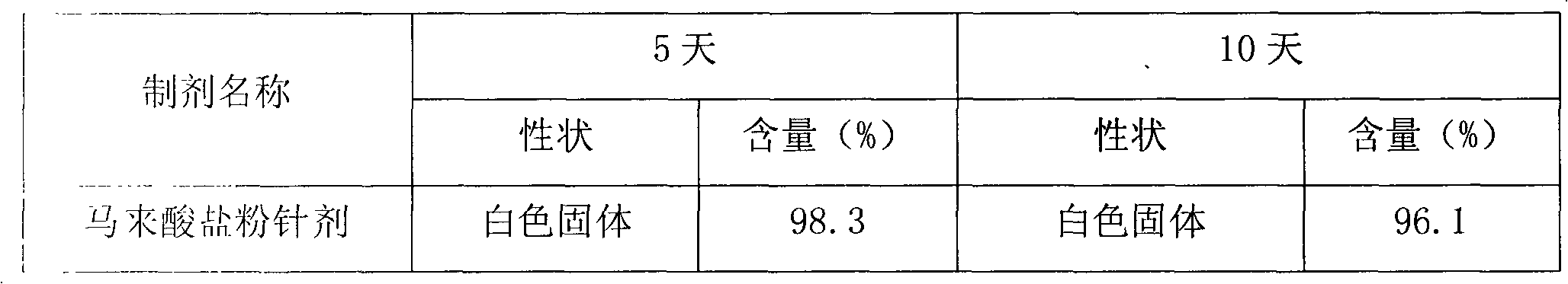
Cinepazide acid addition salt and preparation method thereof
A technology of cinepazide and cinepazide, which is applied in the field of medicine, can solve the problems of poor photostability, inconvenient drug production, storage, transportation and use, hidden dangers of clinical safety medication and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] The preparation of embodiment 1 gluconate
[0083] Dissolve 20 g of cinepazide in 100 ml of ethanol, add 30% gluconic acid ethanol solution dropwise under stirring until the pH value is about 3, slowly add petroleum ether dropwise, continue stirring for 1 hour, filter, and wash with ethanol three times, Dry to obtain gluconate 23.9g, yield 82%.
[0084] 1 H-NMR (DMSO) δ: 2.03 (m, 4H), 2.72 (m, 4H), 3.12 (m, 4H), 3.32 (s, 2H), 3.39 (m, 3H), 3.51 (m, 4H), 3.69 (m, 2H), 3.74 (s, 3H), 3.78 (s, 6H), 6.29 (s, 2H), 7.09 (d, 1H), 7.26 (d, 1H), 10.38 (brs, 1H).
Embodiment 2
[0085] The preparation method of embodiment 2 malate
[0086] Dissolve 20 g of cinepazide in 100 ml of ethanol, add 30% malic acid ethanol solution dropwise under stirring until the pH value is about 3, slowly add petroleum ether dropwise, continue stirring for 1 hour, filter, and wash with ethanol three times, Dry to obtain malate 20.8g, yield 79%.
[0087] 1 H NMR (DMSO) δ: 2.01 (m, 4H), 2.04 (t, 1H), 2.09 (d, 2H), 2.68 (m, 4H), 3.09 (m, 4H), 3.25 (s, 1H), 3.30 (s, 2H), 3.49 (m, 4H), 3.69 (s, 3H), 3.79 (s, 6H), 6.27 (s, 2H), 7.03 (d, 1H), 7.25 (d, 1H), 10.35 ( brs, 2H).
Embodiment 3
[0088] The preparation method of embodiment 3 hydroxymethanesulfonate
[0089] Dissolve 20 g of cinepazide in 100 ml of ethanol, add 30% ethanol solution of hydroxymethanesulfonate dropwise under stirring until the pH value is about 2, slowly add petroleum ether dropwise, continue stirring for 1 hour, filter, and wash with ethanol After drying for three times, 18.5 g of hydroxymethanesulfonate was obtained, with a yield of 73%.
[0090] 1 HNMR (DMSO) δ: 2.04 (m, 4H), 2.56 (s, 2H), 2.77 (m, 4H), 3.15 (m, 4H), 3.34 (s, 2H), 3.49 (m, 4H), 3.80 ( s, 3H), 3.81 (s, 6H), 6.32 (s, 2H), 7.16 (d, 1H), 7.30 (d, 1H), 10.69 (brs, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com