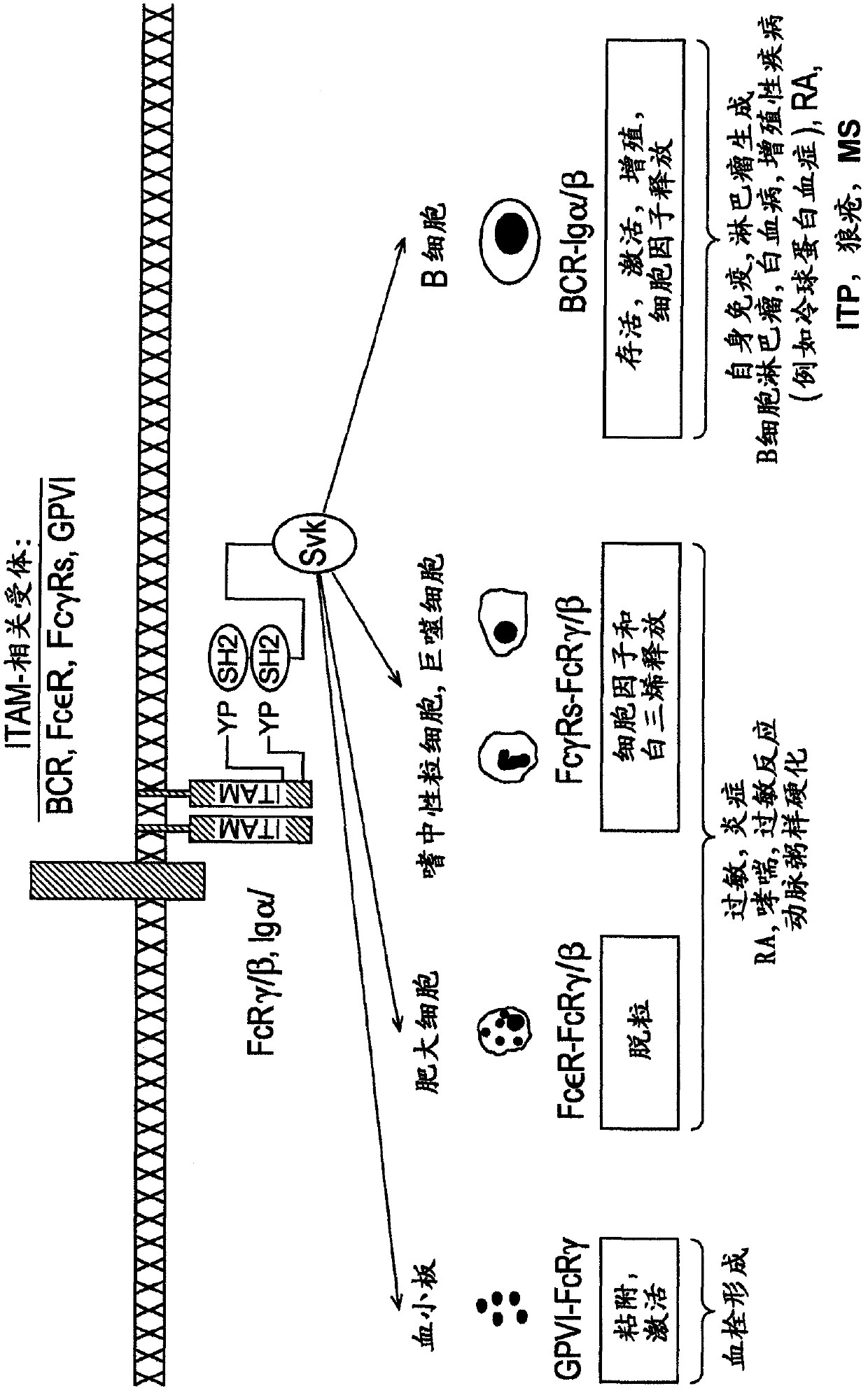
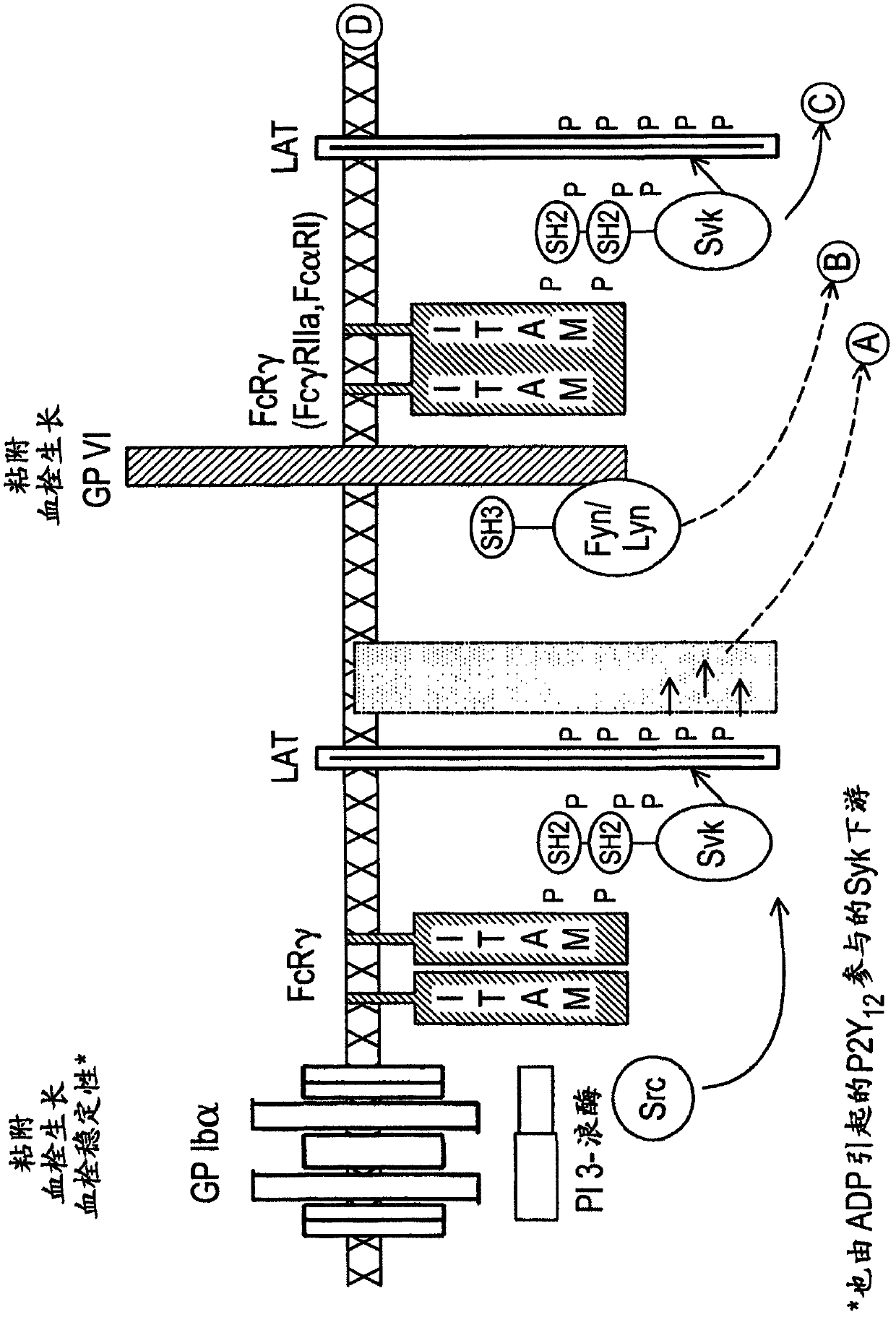
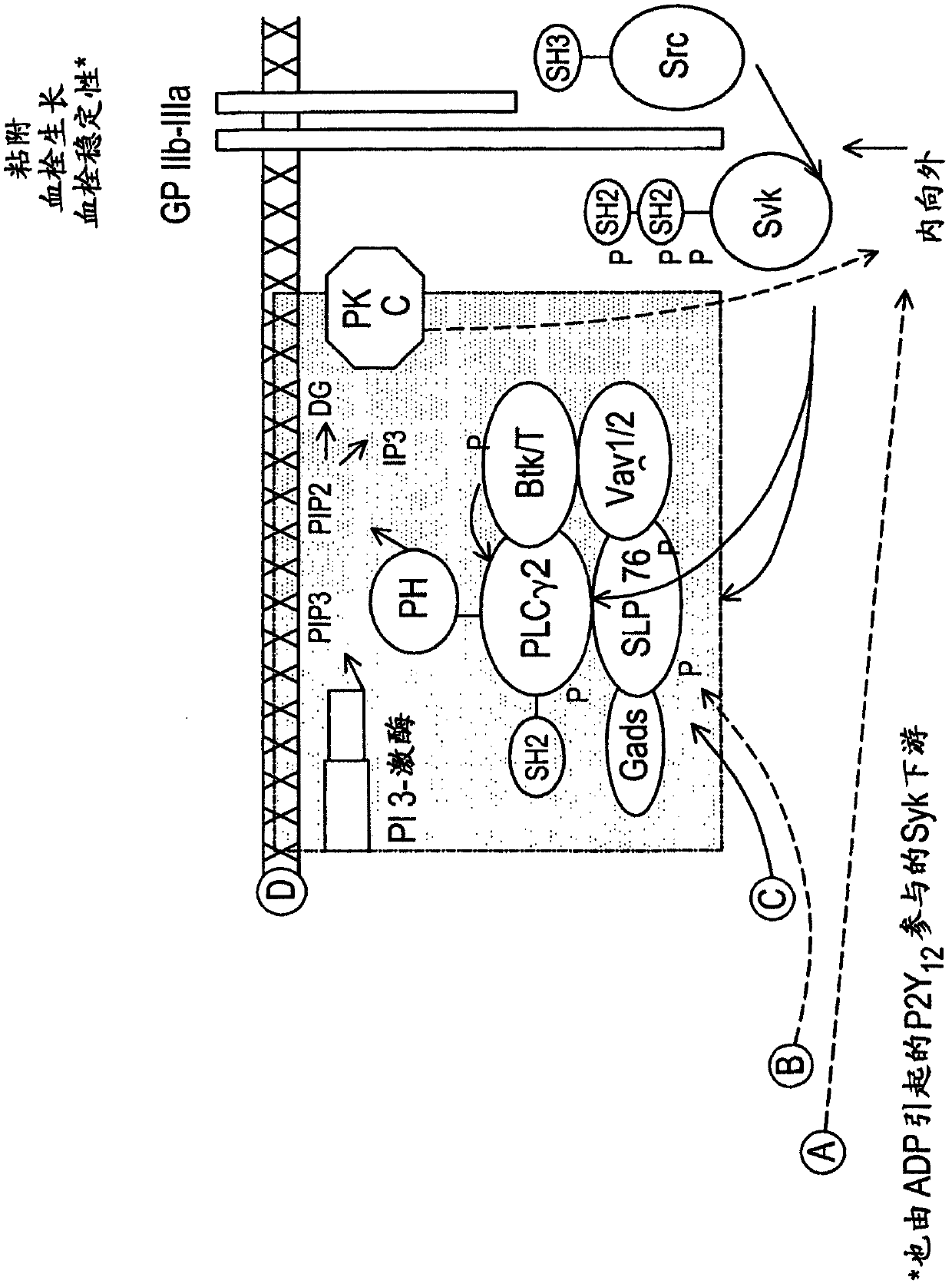
2, 6-diamino-pyrimidin- 5-yl-carboxamides as SRK or JAK kinases inhibitors
A compound, aminocarbonyl technology, applied in anti-inflammatory agents, drug combinations, organic chemistry, etc., can solve the problem of Src kinase inactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0216] a. Compound
[0217] In one embodiment, the present invention provides a compound of formula I or a pharmaceutically acceptable salt thereof:
[0218]
[0219] in:
[0220] D. 1 selected from the following groups:
[0221] (a)C 3-8 Cycloalkyl, which is optionally substituted by 1-4 substituents independently selected from the following groups: C 1-8 Alkyl, amino, hydroxyl, C 1-8 Alkylcarbonyl, aminocarbonyl, C 1-8 Alkoxycarbonylamino, aryl C 1-8 Alkoxycarbonylamino, phenyl and heterocyclyl C 1-8 alkylene;
[0222] (b)C 1-8 Alkyl; which is optionally substituted by 1-4 substituents independently selected from the following groups: amino, oxo, C 1-8 Alkoxy, C 2-8 Alkynyl, cyano, aminocarbonyl, C 1-8 Haloalkyl, Hydroxy, Halogen, C3-8 Cycloalkyl and phenyl;
[0223] (c)C 1-8 Alkyl C 3-8 Heterocyclyl, which is optionally substituted by 1-4 substituents independently selected from the following groups: C 1-8 Alkyl, C 1-8 Alkylcarbonyl, C 1-8 Alkylsulfonyl...
Embodiment 1
[0525] Example 1.2-(4-(4-acetylpiperazin-1-yl)phenylamino)-4-(cyclobutylamino)pyrimidine-5-carboxamide 1
[0526] Process 1:
[0527]
[0528] Step 1: To a stirred solution of carboxylic acid 1.1 (85 g, 540 mmol) in thionyl chloride (425 mL) was slowly added pyridine (8.5 mL, 0.11 mmol). The reaction was stirred overnight at 75 °C, then it was concentrated and dried in vacuo to obtain a pale yellow powder which was used immediately in the next step.
[0529] Step 2: The yellow solid obtained in the previous step was slowly diluted with 750 mL of ethanol and refluxed overnight. The next day, the reaction was complete as determined by HPLC, then cooled in an ice bath, and the solid was filtered and washed with ether to afford the desired ethyl ester (1.3) as an off-white powder (91 g, 87% for two steps). C 7 h 8 N 2 o 4 The MS measured value is (M+H) + 185.0.
[0530] Step 3: Ester 1.3 (22g, 120mmol) was dissolved in phosphorus oxychloride (60mL, 600mmol), the mixt...
Embodiment 32
[0541] Example 32.2,4-bis(4-(4-acetylpiperazin-1-yl)phenylamino)pyrimidine-5-carboxamide
[0542] Process 2:
[0543]
[0544] Step 1: Compound 1.4 (Example 1, 1.05 g, 4.8 mmol) was dissolved in 40 mL of acetonitrile. Sodium thiomethoxide (0.74 g, 10.5 mmol) was added thereto. It was stirred overnight, diluted with ethyl acetate, washed 3 times with brine, dried and concentrated in vacuo. It was then placed in 20 mL of dioxane and 10 mL of water. 500 mg of LiOH hydrate was added thereto. The mixture was stirred for 4 hours. 1N HCl was added to the mixture until the pH reached 3. It was concentrated and extracted 3 times with ethyl acetate. The organic phases were combined, dried and concentrated in vacuo to obtain a white solid. The solid was then dissolved in 30 mL of anhydrous DMF. EDC hydrochloride (1.10 g, 5.7 mmol) and HOBt (0.77 g, 5.7 mmol) were added thereto. The mixture was stirred for 30 min, to which was added ammonia (obtained from a commercial 0.5N sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com