Novel method for extracting lithium from salt lake brine
A technology for extracting lithium from salt lake brine, applied in the field of lithium extraction, can solve the problems of complicated process and high production cost, and achieve the effect of simple process operation and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] Cation exchange resin: Xi'an Lanxiao Technology Co., Ltd., gel type strong acid cation exchange resin LSD-010.
[0026] Resin magnesium removal steps:
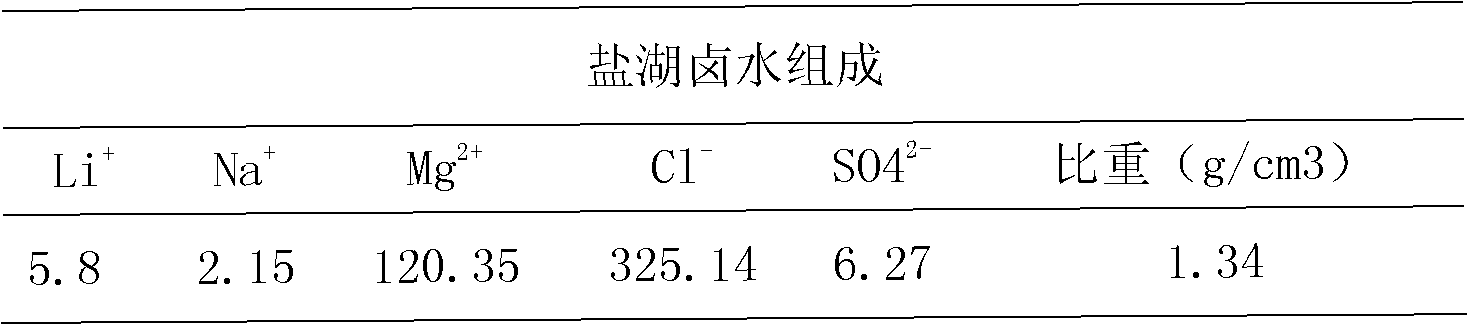
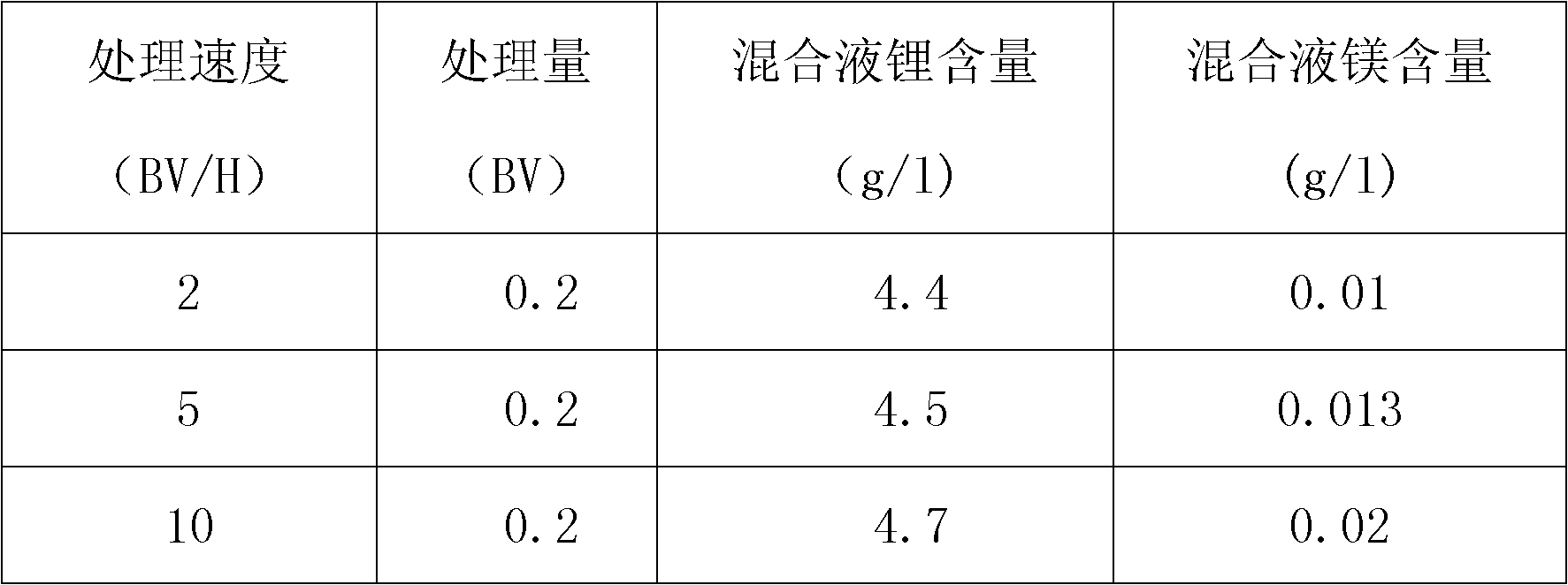
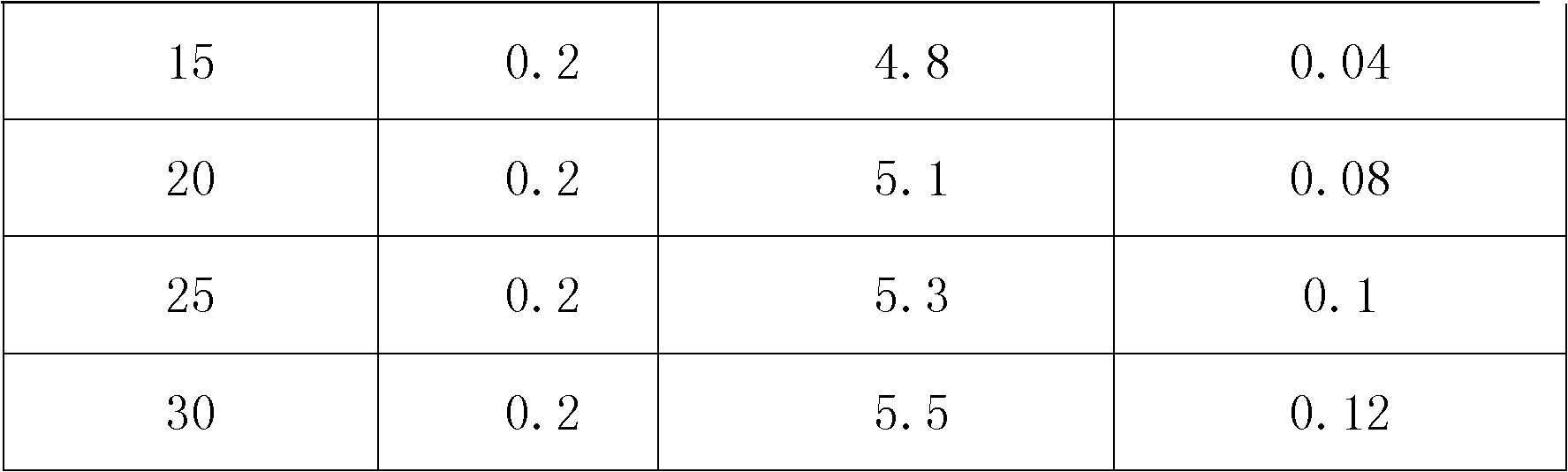
[0027] Put the cation exchange resin into a Φ30*1000mm plexiglass column, pass it into the salt lake brine, pass through the gel-type strong acid cation exchange resin column at a flow rate of 5BV / H, treat 0.1BV, and take the effluent to detect the content of magnesium and lithium by atomic absorption method Respectively: 0.002g / L and 4.8g / L, the lithium ion content of the effluent is concentrated by a reverse osmosis membrane to reach 25g / l, and solid sodium carbonate is added to precipitate lithium carbonate.
[0028] The ratio of magnesium to lithium in the concentrated brine is Mg / Li<350.
Embodiment 2
[0030] The salt lake brine comes from Taijier Lake in Qinghai, and the composition of the salt lake brine is the same as in Example 1;
[0031] Cation exchange resin: Xi'an Lanxiao Technology Co., Ltd., macroporous strong acid cation exchange resin D001.
[0032] Resin pretreatment: 7% Nacl solution for 3 resin bed volumes, washed with water until the conductivity is less than 50us.
[0033] Resin magnesium removal steps:
[0034] Put the cation exchange resin into a Φ30*1000mm plexiglass column, pass it into the salt lake brine, pass through the macroporous strong acid cation exchange resin column at a flow rate of 5BV / H, treat 0.2BV, and take the effluent to detect the content of magnesium and lithium by atomic absorption method, respectively 0.055g / L and 5.1g / L, the lithium ion content of the effluent is concentrated by reverse osmosis membrane to reach 25g / l, and solid sodium carbonate is added to precipitate lithium carbonate.
Embodiment 3
[0036] The salt lake brine comes from Taijier Lake in Qinghai, and the composition of the salt lake brine is the same as in Example 1;
[0037] Cation exchange resin: Xi'an Lanxiao Technology Co., Ltd., macroporous strong acid cation exchange resin SEPLITE SC-20.
[0038] Resin pretreatment: 7% Nacl solution for 3 resin bed volumes, washed with water until the conductivity is less than 50us.
[0039] Resin magnesium removal step: put the cation exchange resin into a Φ30*1000mm plexiglass column, pass it into the salt lake brine, pass through the macroporous strong acid cation exchange resin column at a flow rate of 5BV / H, treat 0.3BV, and take the effluent to detect magnesium by atomic absorption method Lithium content and lithium content are respectively: 6.7g / L and 5.4g / L, described effluent is concentrated lithium ion content reaches 25g / l through reverse osmosis membrane, adds solid sodium carbonate, precipitates out lithium carbonate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com