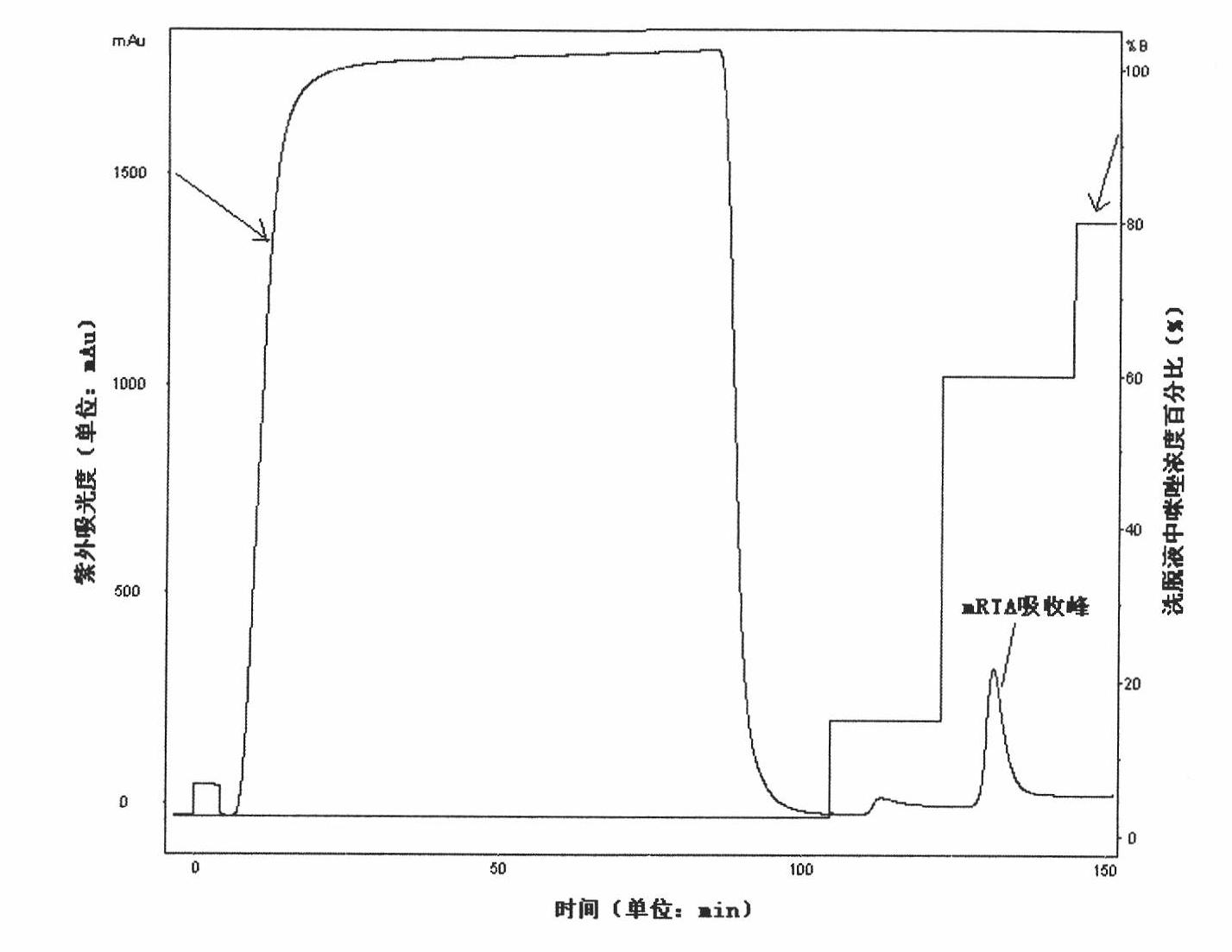
Construction of recin A chain mutant and application of recin A chain mutant as candidate vaccine antigen
A technology of vaccines and recombinant vectors, applied in antiviral agents, recombinant DNA technology, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, the acquisition of mutant mRTA
[0031] 1. Obtaining of mutant mRTA
[0032] According to the A chain (rRTA) gene sequence of existing ricin, its nucleotide sequence is the sequence 5 in the sequence listing, and the aminoacid sequence is the sequence 6 in the sequence listing, adopts Quickchange Lighting Site-Directed Mutagenesis Kit (Stratagene company , Catalog#210518) to obtain mutant mRTA-D75AV76MY80A (plasmid) by site-directed mutation, after sequencing, the gene contained in mRTA-D75AV76MY80A was named mRTA, its nucleotide sequence was sequence 1 in the sequence listing, and the coding region was sequence 1 in the sequence listing The 1st-801st nucleotide sequence from the 5' end of sequence 1, the amino acid sequence of mRTA protein encoded by mRTA is the sequence shown in sequence 2 in the sequence list. Using Lasergene software to predict the antigenicity of the constructed mutants did not change significantly.
[0033] The mutation results wer...
Embodiment 2
[0047] Embodiment 2, the biological activity detection of mutant mRTA
[0048] 1. Quantification of Mutant Proteins
[0049] The purified mRTA obtained in Example 1 was quantified using the BCA method protein quantification kit (purchased from Beijing Biotech Biotechnology Co., Ltd., Lot #20120613). The experiment was repeated three times, and the results were averaged.
[0050] Specific steps:
[0051] Kit composition: protein standard (5mg / mlBSA), Solution A, Solution B.
[0052] (1) When using, shake and mix Solution A. According to the number of samples, prepare an appropriate amount of BCA working solution with 50 volumes of Solution A plus 1 volume of Solution B (50:1), and mix well.
[0053] (2) Completely dissolve the protein standard (5mg / mlBSA), dilute it to 1.5mg / ml, 1.0mg / ml, 0.8mg / ml, 0.4mg / ml, 0.2mg / ml with PBS as the standard.
[0054] (3) Add the standard substance and the sample to be tested at each concentration mentioned above to the 96-well plate respect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com