Liver cancer multi-marker micro-array kit as well as preparation method and application thereof
A multi-marker, microarray technology, applied in the biological field, can solve problems such as difficult to distinguish benign from malignant lesions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Quality detection of the chemiluminescent quantitative enzyme-linked immunoassay kit of the present invention
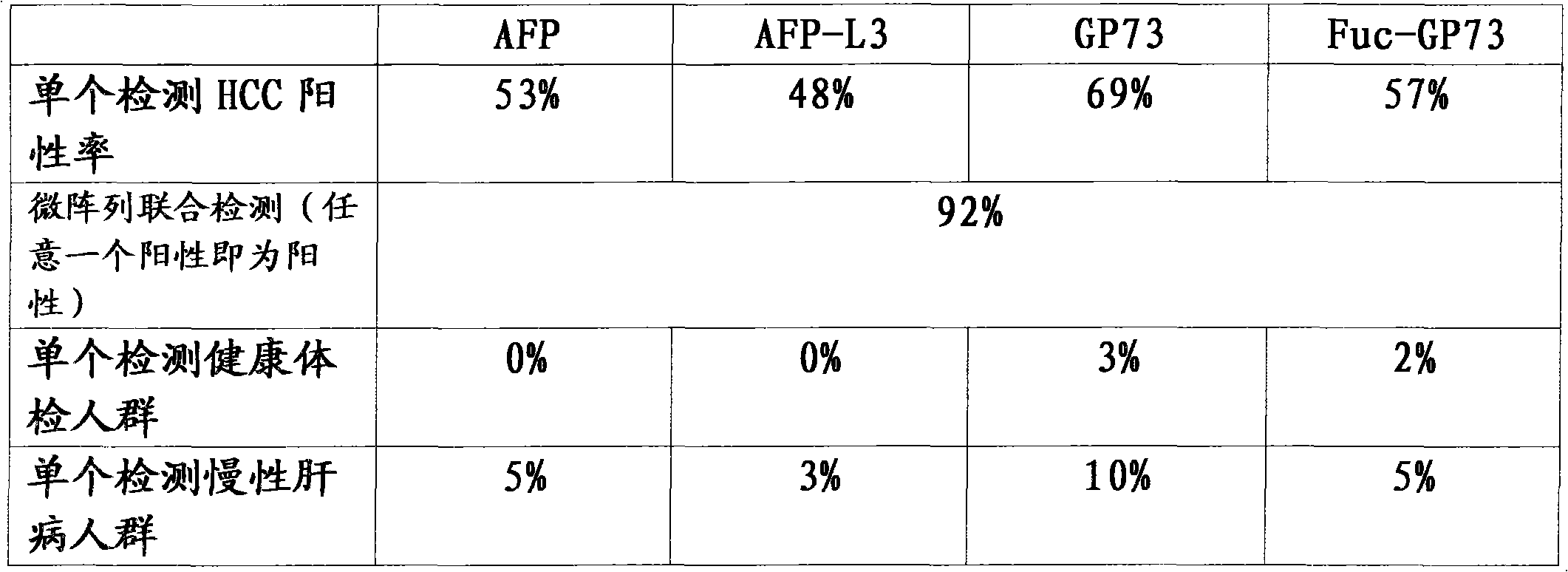
[0046] 1) Accuracy: In the test results of 15 negative quality control sera (including specific control sera) in normal physical examination, no false positives occurred. There were no false negatives in the test results of 10 reference samples of liver cancer HCC positive quality control serum.
[0047] 2) Precision: 20 boxes of different batches of kits were randomly selected, and the same HCC antigen-positive quality control serum was used for repeated measurements according to the instructions. Calculate the results of each measurement, find the mean, SD and coefficient of variation CV. The results of the precision test showed that the CV between batches was <15%.
[0048] 3) Detection sensitivity: the detection sensitivity of this kit is 5ng / ml.
[0049] 4) Specificity: Take four samples to be tested and mix them into four mixed serum sample...
Embodiment 2
[0051] Example 2: Preparation of liver cancer multi-marker microarray chemiluminescence kit
[0052] This embodiment prepared a liver cancer multi-marker microarray chemiluminescence kit (96 servings) of the present invention, which consists of:
[0053] AFP / GP73 monoclonal antibody coated plate (96 wells) 1 piece;
[0054] 1 bottle of horseradish peroxidase (HRP)-labeled AFP antibody, 1 bottle of horseradish peroxidase (HRP)-labeled GP73 polyclonal antibody, 1 bottle of horseradish peroxidase (HRP)-labeled lectin LCA solution , respectively 10ml / bottle;
[0055] 1 bottle of substrate solution: EDTA (1.0×10 -2 M), H 2 o 2 (7.5×10 -3 M), HCl (1.0×10 -2 M) and Tween20 (1%)
[0056] Chromogenic solution 1 bottle: luminol 5.0×10 -4 Mol / L;
[0057] 1 bottle of sample diluent: 20mM pH 7.4 PBS buffer containing 2% BSA
[0058] The specific operation is as follows:
[0059] 1. Make AFP / GP73 monoclonal antibody coated plate:
[0060] The enzyme plate adopts the 12×8 detacha...
Embodiment 3
[0066] Example 3: The method of using the multi-marker microarray chemiluminescent immunoassay kit for liver cancer
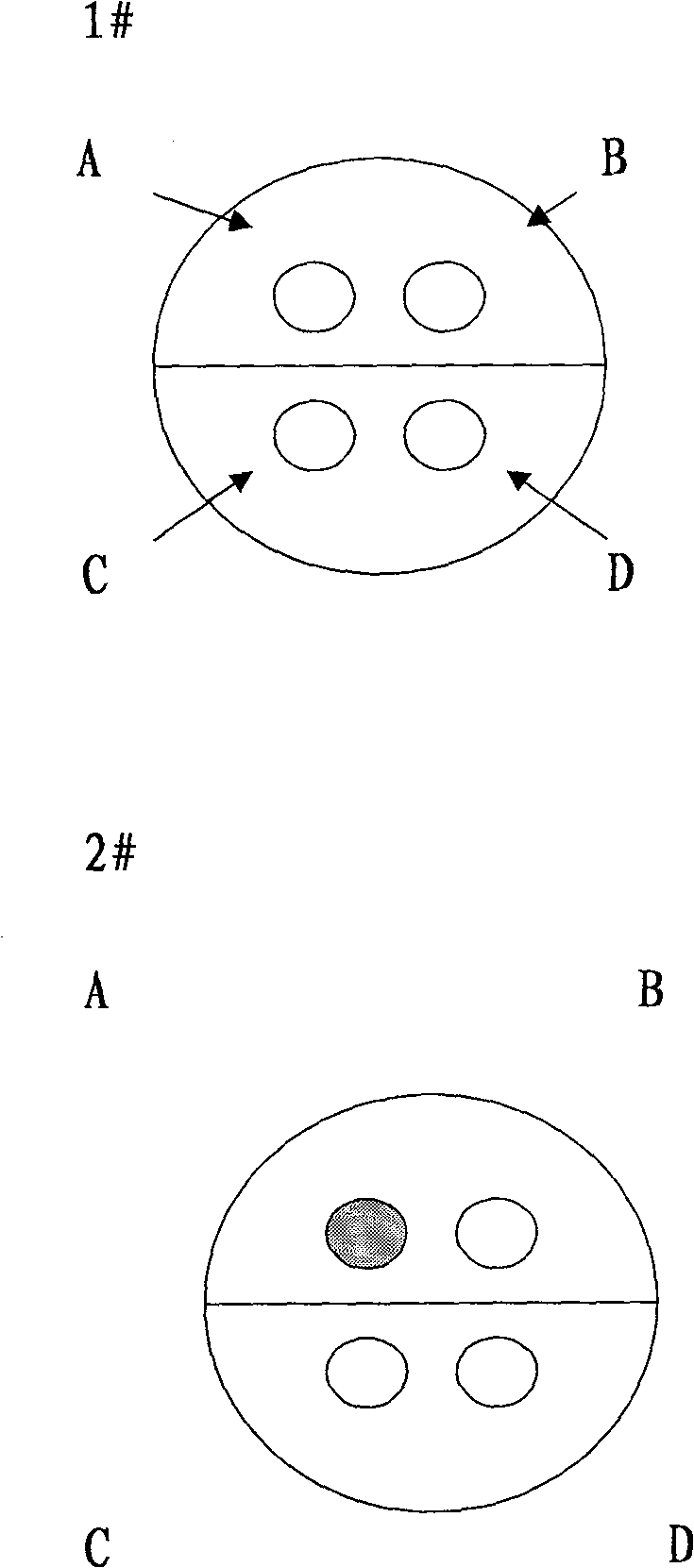
[0067] a) Monoclonal antibody capture antigen reaction: add 100 μl sample diluent to the corresponding microwells of the antibody-coated plate provided in the kit, then add 10 μl serum sample to be tested, incubate in a 37°C water bath for 30 minutes, and wash the plate. A total of 4 well reactions, respectively Al, Cl, El, Gl;
[0068]d) Add AFP-mAb-HRP solution to Al, add LCA-HRP solution to Cl well, add GP73 mAb-HRP solution to El well, 100 μl per well, and keep warm in 37° C. water bath for 30 minutes. Repeat the plate washing operation 4 times.
[0069] c) Chromogenic reaction: add substrate solution A and 50 μl of chromogenic solution B to each well successively, and read the value.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com